
- 232 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
The basics of group theory and its applications to themes such as the analysis of vibrational spectra and molecular orbital theory are essential knowledge for the undergraduate student of inorganic chemistry. The second edition of Group Theory for Chemists uses diagrams and problem-solving to help students test and improve their understanding, including a new section on the application of group theory to electronic spectroscopy.Part one covers the essentials of symmetry and group theory, including symmetry, point groups and representations. Part two deals with the application of group theory to vibrational spectroscopy, with chapters covering topics such as reducible representations and techniques of vibrational spectroscopy. In part three, group theory as applied to structure and bonding is considered, with chapters on the fundamentals of molecular orbital theory, octahedral complexes and ferrocene among other topics. Additionally in the second edition, part four focuses on the application of group theory to electronic spectroscopy, covering symmetry and selection rules, terms and configurations and d-d spectra.Drawing on the author's extensive experience teaching group theory to undergraduates, Group Theory for Chemists provides a focused and comprehensive study of group theory and its applications which is invaluable to the student of chemistry as well as those in related fields seeking an introduction to the topic.
- Provides a focused and comprehensive study of group theory and its applications, an invaluable resource to students of chemistry as well as those in related fields seeking an introduction to the topic
- Presents diagrams and problem-solving exercises to help students improve their understanding, including a new section on the application of group theory to electronic spectroscopy
- Reviews the essentials of symmetry and group theory, including symmetry, point groups and representations and the application of group theory to vibrational spectroscopy
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Group Theory for Chemists by Kieran C Molloy in PDF and/or ePUB format, as well as other popular books in Mathematics & Group Theory. We have over one million books available in our catalogue for you to explore.
Information
Part I
Symmetry and Groups
1
Symmetry
While everyone can appreciate the appearance of symmetry in an object, it is not so obvious how to classify it. The amide (1) is less symmetric than either ammonia or borane, but which of ammonia or borane – both clearly “symmetric” molecules – is the more symmetric ? In (1) the single N-H bond is clearly unique, but how do the three N-H bonds in ammonia behave ? Individually or as a group ? If as a group, how ? Does the different symmetry of borane mean that the three B-H bonds will behave differently from the three N-H bonds in ammonia ? Intuitively we would say “yes”, but can these differences be predicted ?
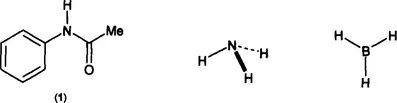
This opening chapter will describe ways in which the symmetry of a molecule can be classified (symmetry elements and symmetry operations) and also to introduce a shorthand notation which embraces all the symmetry inherent in a molecule (a point group symbol).
1.1 SYMMETRY
Imagine rotating an equilateral triangle about an axis running through its midpoint, by 120° (overleaf). The triangle that we now see is different from the original, but unless we label the corners of the triangle so we can follow their movement, it is indistinguishable from the original.
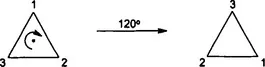
The symmetry inherent in an object allows it to be moved and still leave it looking unchanged. We define such movements as symmetry operations, e.g. a rotation, and each symmetry operation must be performed with respect to a symmetry element, which in this case is the rotation axis through the mid-point of the triangle.
It is these symmetry elements and symmetry operations which we will use to classify the symmetry of a molecule and there are four symmetry element ⁄ operation pairs that need to be recognised.
1.1.1 Rotations and Rotation Axes
In order to bring these ideas of symmetry into the molecular realm, we can replace the triangle by the molecule BF3, which valence-shell electron-pair repulsion theory (VSEPR) correctly predicts has a trigonal planar shape; the fluorine atoms are labelled only so we can track their movement. If we rotate the molecule through 120° about an axis perpendicular to the plane of the molecule and passing through the boron, then, although the fluorine atoms have moved, the resulting molecule is indistinguishable from the original. We could equally rotate through 240°, while a rotation through 360° brings the molecule back to its starting position. Each of these rotations is a symmetry operation and the symmetry element is the rotation axis passing through boron.
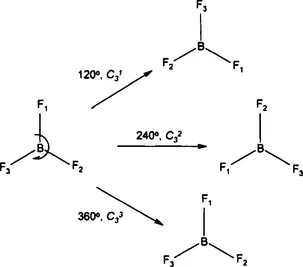
Fig. 1.1 Rotation as a symmetry operation.
Remember, all symmetry operations must be carried out with respect to a symmetry element. The symmetry element, in this case the rotation axis, is called a three-fold axis and is given the symbol C3. The three operations, rotating about 120°, 240° or 360°, are given the symbols C31, C32 and C33, respectively. The operations C31 and C32 leave the molecule indistinguishable from the original, while only C33 leaves it identical. These two scenarios are, however, treated equally for identifying symmetry.
In general, an n-fold Cn axis generates n symmetry operations corresponding to rotations through multiples...
Table of contents
- Cover image
- Title page
- Table of Contents
- About the Author
- Copyright
- Preface
- Part I: Symmetry and Groups
- Part II: Application of Group Theory to Vibrational Spectroscopy
- Part III: Application of Group Theory to Structure and Bonding
- Part IV: Application of Group Theory to Electronic Spectroscopy
- Appendices
- Index