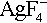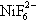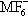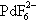![]()
This book reflects the achievements and challenges for the field of Fluorine Chemistry at the turn of the millennium. Many of the most active participants in that chemistry have contributed chapters on their particular specialties. These remarks can only give the slightest sketch of the overall situation. For an authoritative and accurate assessment of each aspect of the field, the reader must turn to the appropriate chapter
The small size and high electronegativity of the F-ligand, combined with the low bond energy in F2 and the strong bonds that the ligand makes with most other atoms, are the fundamental characteristics that lie at the basis of the ready combustion of most of the elements in fluorine, often resulting in their oxidation to the highest known state of oxidation. Fluoride synthesis in the past, at least in its inorganic aspects, was often simply a matter of heating materials in F2 (sometimes under pressure). Today’s picture is changed dramatically.
Perhaps the ready availability of translucent fluorocarbon plastics such as FEP, which has remarkable kinetic stability to F
2, has been the major technical factor in providing for low temperature synthesis of fluorides, especially in the highest attainable oxidation-states. The use of anhydrous liquid hydrogen fluoride, aHF, in such apparatus has provided not only an excellent ionizing solvent with a long liquid range, but also one that is remarkably robust oxidatively and reductively. In addition, the transparency of the FEP plastics to visible and near-UV light, has provided for the effective photo-dissociation of F
2. A flux of F atoms generated in this way, in aHF made basic with good fluoride-ion donors (such as alkali fluorides), has provided high oxidation-state species, such as
and
, at room temperature. From such salts as these, at temperatures below 20 °C, AgF
3, NiF
4, NiF
3, and other fluorides, thermodynamically unstable (but kinetically stable) with respect to loss of F
2, have been prepared, and characterized. These have been used as potent fluorinating and oxidizing agents, e.g. cationic Ag(III) or Ni(IV) in aHF, at ~ 20 °C, efficiently generate MF
6 (M = Pt, Ru, Rh) from
.
Low-valent transition-element fluorides, especially those in which carbonyl and organo-ligands are incorporated, require other approaches, which are tailored to the element and the oxidation state. Here an inherent thermodynamic problem is imposed by the high C—F bond energy, which can exceed the M—F bond energy, never-the-less an increased range of such materials is in prospect. Perfluorinated organic solvents evidently have a valuable role to play in this organo-metallic fluorine chemistry, where their redox and substitutional inertness are especially important.
The solvent aHF (because it is not easily reduced) is also likely to have an important role in the future in low oxidation-state chemistry. This solvent is a super-acid and in keeping with that character, low oxidation-state transition-metal cations, including carbonyl cations of such metals, can be prepared. Indeed, since the electronegativity of a given oxidation state must be higher in a cation than in the neutral or anionic relatives, it follows that low oxidation states are more likely to be achieved if oxidations (fluorinations) are carried out in solutions made acidic with strong fluoride-ion acceptors such as SbF
5. Conversely, when the ionizing solvent (e.g. aHF) favors anion formation (as when alkali fluoride is present in aHF) high oxidation states result. So we see that oxidation of Pd metal with F
2 in acidified aHF gives a cationic Pd(II) solution from which PdF
2 may be precipitated, whereas Pd metal with F
2 in aHF containing alkali fluoride yields
from which PdF
4 can be displaced with AsF
5. The synthetic approaches discussed so far, however, are not efficiently applicable to oxyfluorides.
Efficient synthesis of an oxyfluoride often proceeds from an oxide with substitution of two F-ligands for one O-ligand. This requires a reagent which makes very strong bonds to oxygen. Especially useful are SF4, PF5, and XeF6. Since the last is not a reducing agent and can also be used as a solvent it is valuable in the generation of high oxidation-state oxyfluorides as exemplified by a variety of new technetium and rhenium oxyfluoro species. Good synthetic routes to main-group oxyfluorides, particularly those with OSO2F, and OTeF5 groups are presently of special interest as ligands of anions that have only very weak interaction with any cation (so-called naked cation). A variety of main-group and transition-element derivatives of the OTeF5 group, are described here.
The strong bonding of the fluorine ligand to most other atoms has given rise to a major interest in fluoro-coatings for surfaces and this has in turn fostered much work on the effective use of plasmas in which SF6 or CF4 other kinetically inert (and hence rather safe reagents) are used as the source of F atoms. Such fluorocoatings can profoundly affect not only the susceptibility of the material to chemical attack but also the physical behavior, such as wettability and lubricity.
The need to replace chlorofluorocarbons and molecules related to them, because of the damage they do to the earth’s protective ozone shield, has presented organo-fluorine chemists with the challenges of finding replacements for these widely used and economically important molecules. An aspect of the fluorination chemistry involved in the synthesis of substitutes, commonly involving the conversion of C1 and C2 compounds is the best choice of heterogeneous catalyst. Commonly such catalysts are fluorinated chromia or alumina. Their possible mechanistic roles have been investigated and point to ways in which fluorination specificity and efficiency might be improved.
As a consequence of the monovalence of fluorine, fluorides at the high oxidation-state limit are often at, or near, the coordination limit for that element, with the result that such binary fluorides are easily volatile. This provides convenient gaseous sources for many metals (TiF4, WF6, MoF6, PtF6, are examples) some of which have already found applications in the semi-conductor industry. In lower oxidation states the commonly found six coordination is usually maintained, with an appropriate number of F-ligands symmetrically linking two metal centers. These μ-fluoro bridges usually have M—F interatomic distances that are ~ 0.2 Å longer than the non-bridging, but these are nevertheless strong enough to give many polymeric fluorides high thermal stability. Fluoride glasses and many other solid-state fluoro-materials derive their utility from such favorable bridge-bonding.
Fluoride glasses owe many of their optical properties to the high electronegativity of the F-ligand. An instance is the positioning of ligand-to-metal charge-transfer bands well into the UV. They have many potential applications, e.g. as lasers, optical fibers, waveguides, and optical amplifiers.
The luminescent properties of crystalline fluorides have also proved to be valuable, especially for certain lasers and scintillators.
The building of μ-fluoro bridged networks about removable template molecules has been known now for a number of years, most simply in the tungsten-bronze form trifluorides of the first transition series. This has motivated a synthetic program to combine this experience with the well known ability of oxides (particularly silicates) to form open frameworks, with resultant microporosity. Because silicates can generate SiF4 in a fluorine rich environment, attention is particularly given to AlPO4 and GaPO4 based solids.
In. addition to strong bonding, the M—F—M bridges also provide for moderately strong antiferromagnetic coupling of the magnetic centers at each M, unless the M—F—M angle is close to 90°. The antiferromagnetic coupling is frustrated when three M are linked in a ring. When the magnetic centers are inequivalent the antiferromagnetic coupling results in field dependence (ferrimagnetism). There is therefore a rich variety of magnetic behavior in transition-metal fluorides that is nicely illustrated by the usovite and jarlite relatives described here.
The electronegative character of fluorine invites its application, as an agent of energy storage, in combination with the electropositive element lithium. So far, the most useful approximations to this system have been via the lithium/graphite fluoride combination. Indeed, this has long been the basis of an important primary battery. It is evident that the lamella character of the graphite fluorides (whether they are flat carbon sheets, sp2, or puckered, sp3) is probably crucial for the required facile migration of F from the graphite fluoride electrode to the electrolyte. Reconstitution of the graphite π system as F is removed could also be beneficial to the discharge process (and probably essential for a reversible electrode). There is an urgent need, for a secondary battery based on such lightweight high energy-density materials. This requires reversible lithium and fluorine source electrodes and a compatible electrolyte. Chapters in this volume address these difficult problems, and potential raw material sources for the graphite fluorides.
That the F-ligand can attach to the C atoms of graphite without affecting the sp2 orbital hybridization has long been known, and it is now clear from the study of the fluorination products of the fullerenes, that attachment of F-ligand to the C atoms of these molecules does not have profound influence on the C orbital hybridization either. The impact of fluorination on the physical and chemical properties of these new forms of carbon can now be seen.
Much of the work done in Fluorine Chemistry is financed or motivated from the economic benefits of industrial applications, many of which are outlined in the final two chapters of this volume. But, as we all know, th...