- 1,088 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Applied Polymer Science: 21st Century
About this book
The 75th Anniversary Celebration of the Division of Polymeric Materials: Science and Engineering of the American Chemical Society, in 1999 sparked this third edition of Applied Polymer Science with emphasis on the developments of the last few years and a serious look at the challenges and expectations of the 21st Century.This book is divided into six sections, each with an Associate Editor responsible for the contents with the group of Associate Editors acting as a board to interweave and interconnect various topics and to insure complete coverage. These areas represent both traditional areas and emerging areas, but always with coverage that is timely. The areas and associated chapters represent vistas where PMSE and its members have made and are continuing to make vital contributions. The authors are leaders in their fields and have graciously donated their efforts to encourage the scientists of the next 75 years to further contribute to the well being of the society in which we all live.Synthesis, characterization, and application are three of the legs that hold up a steady table. The fourth is creativity. Each of the three strong legs are present in this book with creativity present as the authors were asked to look forward in predicting areas in need of work and potential applications. The book begins with an introductory history chapter introducing readers to PMSE. The second chapter introduces the very basic science, terms and concepts critical to polymer science and technology. Sections two, three and four focus on application areas emphasizing emerging trends and applications. Section five emphasizes the essential areas of characterization. Section six contains chapters focusing of the synthesis of the materials.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Applied Polymer Science: 21st Century by C. Craver,C. Carraher in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Materials Science. We have over one million books available in our catalogue for you to explore.
Information
POLYMER SCIENCE AND TECHNOLOGY
David J. Lohse, Section Editor
POLYMER CHAIN CONFIGURATIONS: MEASUREMENT AND APPLICATIONS
LEWIS J. FETTERS, Corporate Research Labs, Exxon Research & Engineering Co., Rte. 22 East, Annandale, NJ 08801-0998
Outline
Introduction
Unperturbed Chain Dimensions
The Packing Length Influence in Linear Polymer Melts
Applications
Outline
A primary goal of polymer science has been to relate macromolecular structure to macroscopic properties. In particular, it has been hoped that the sizes of polymer coils could be related to the degree with which they entangle, and hence, to their viscoelasticity in the melt. This notion has been realized via the use of the concept of the packing length (p) which can be defined as the volume of a chain divided by its root mean square end to end distance. This has led to the development of simple correlations between such properties as the chain dimension (<R2>0), density (p) and plateau modulus (GN0). The interplay of these observable parameters leads to GN0 ∝ Tp−3 and Me ∝ ρp3 where Me denotes the entanglement molecular weight. These relations seem to be universal for Gaussian chains in the melt-state and can be extended to include Mc, which marks the onset of entanglement effects, and Mr, the crossover to the reptation form. These expressions are useful for their predictive powers. A practical example in the design of adhesives is given.
Introduction
In 1952 Bueche (1) proposed that the melt viscosity of short polymer chains (pre-entanglement regime) could be expressed as follows:
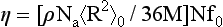
Here, the observable parameters are the chain density (ρ), the Avogadro number (Na), the chain molecular weight M, the unperturbed chain dimension (<R2>0) expressed in terms of the root-mean-square end-to-end distance, N, the number of backbone atoms and f0 the molecular friction factor. Fox and Allen (2) demonstrated the validity of Eq. 1 in 1964.
Bueche (1) also showed that the combination of the melt viscosity and the diffusion constant led to the now well-known relation:
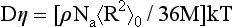
Thus, Bueche’s work was the first to show that density and the unperturbed chain dimension were directly relatable to polymer melt rheological properties.
In 1969 Flory (3) offered the following observation: “Comprehension of the configurational statistics of chain molecules is indispensable for a rational interpretation and understanding of their properties”. The verity of his statement has become more apparent, in the intervening years, with the realization that these chain dimensions were related to the plateau modulus, GN0, of a polymer. Empirically, it was recognized that GN0 values decrease as chain ‘thickness’ increased. For example (4), polyethylene shows GN0 of ∼2.5 MPa, polyisobutylene the value of 0.32 MPa, polystyrene the value of 0.20 MPa and poly(cyclohexyl ethylene) the value of ∼0.07 MPa over the temperature range of 413 to 433 K. This decrease in GN0 is accompanied by a corresponding decrease (ca. four-fold) in the respective unperturbed chain dimensions (4).
The first rigorous attempt to relate Gaussian chain dimensions to GN0 was that of Graessely and Edwards (5). Shortly thereafter Ronca (6) presented the basics of what now is referred to as the packing model. Later contributions to the packing model were made by Lin (7) and Noolandi and Kavassalis (8,9). At the same time Witten, Milner, and Wang (10) introduced the concept of the packing length (p), which is defined as the chain volume divided by the unperturbed mean square chain end-to-end distance. Thus:

where M/ρNa is the inverse of the number of molecules per unit volume. It is apparent that the Bueche equations, (1) and (2), can be recast in terms of the packing length.
The pivotal role in melt rheological behavior of the packing length is discussed below. The importance of these findings is to illustrate the overriding importance of the unperturbed chain dimension in the physical characteristics of melt polymer chains. This article provides information upon the acquisition of this parameter and recent developments (4,11,12) in its use in predicting the viscoelastic parameters of GN0, Me (the entanglement molecular weight), Mc (the critical molecular weight), Mr (the reptation molecular weight) and the entanglement length. Mc and Mr are the molecular weights observed, respectively, at the point where the melt Newtonian viscosity enters the regime where viscosity commences to scale with M3.4 and at which the crossover to the reptation gradient of 3 occurs.
Unperturbed Chain Dimensions
Historically (13), the measure of an unperturbed chain dimension has been carried out in dilute solution where the technique of assay has been via dilute solution viscosity measurements or light scattering. The primary proviso for these evaluations is that the solvent used provides an environment where excluded volume effects are totally screened out at a certain temperature. This is the state that is referred to as the theta condition and at which the second virial coefficient is zero. For a given solvent-polymer pair this state exists at a single temperature. A parallel parameter of interest has been the chain dimension temperature coefficient (14): κ = d(ln <R2>0)/dT. Experimentally, the values found embrace the range of negative to positive. Again, the basic experimental protocols are the same as those involved in the chain dimension evaluation with the added need of finding a series of theta solvents over as broad a temperature range as possible. Such an approach requires the needed assumption that the theta solvents used do not in their own right influence (via specific solvent effects) the necessary unperturbed chain posture. As will be seen later this assumption can be invalid. A second approach to the measurement of κ involves the thermoelastic approach (15,16), a concept that allows the evaluation of κ for chains in the crosslinked melt-state. This involves the thermodynamic quantity, fe, the energetic component of the total elastic force, f, and temperature, T. This in turn leads to the relation (15,16)
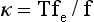
The evaluation of κ in this fashion has the advantage of being done in the bulk state. That is the natural environment of the chain and thus dispenses with the possibility of distortions due to specific solvent effects and the necessity of finding a suitable series of theta solvents useable over a convenient temperature range. However, the absolute value of <R2>>0/M is not obtainable by this technique.
The measurement of <R2>0/M in dilute solution has for the most part used intrinsic viscosity measurements as the analytical tool of choice. Relative to light scattering the viscosity method is simpler and requires relatively inexpensive equipment. However, the method is not an absolute one (3). This is shown in the following:
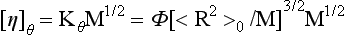

The observable chain dimension parameter, <R2>0/M, is obtainable from ligh...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- PREFACE
- CLARA D. CRAVER
- CHARLES E. CARRAHER Jr.
- INTRODUCTION
- POLYMER SCIENCE AND TECHNOLOGY
- COATINGS
- NEW MATERIALS
- SPECTROSCOPIC & PHYSICAL CHARACTERIZATION
- POLYMERIZATION AND POLYMERIZATION MECHANISMS
- Author Index
- Keyword index