
- 830 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Handbook of Chemical Technology and Pollution Control
About this book
The Handbook of Chemical Technology and Pollution Control, Third Edition provides a detailed review of the chemistry and operating conditions of many of the present large-scale chemical processes important to our economy and high standards of living. The processes that could lead to emissions affecting our air, soil, and water are considered, together with ways in which it may be possible to reduce or eliminate these pollutants. Focusing on cleaner production concepts without neglecting 'end of pipe' measures. With an increase in the awareness of corporate and social responsibility among business and industry leaders, the pressure to reduce harmful emissions and the desire to increase efficiencies and energy utilization, this book provides an essential resource. Suitable for researchers, practitioners and postgraduate students in the fields of chemical and biochemical engineering and environmental science, as well as government monitoring and regulatory agencies and industry leaders who want to stay one step ahead, this book will be a valuable addition to any library.- Integrated treatment of chemical technology with emission control chemistry- Introductory outline of the causes and effects of air and water pollution chemistry- Outline of the operating features and efficiency of basic emission control devices- Historical background of developments in industrial chemistry to 2004 in a single volume- Organized for easy access to chemical technology, new developments, or emission control details- Referenced to current additional sources of information in each area covered- Review questions provide working experience with the material provided
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Background and Technical Aspects
1.1 IMPORTANT GENERAL CHARACTERISTICS
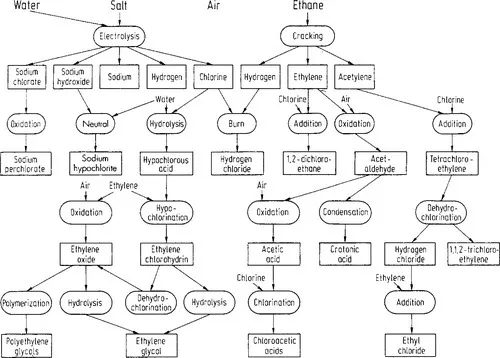
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright page
- Foreword to the Third Edition
- Preface to the Third Edition
- Preface to the Second Edition
- Preface to the First Edition
- Acknowledgments
- 1: Background and Technical Aspects
- 2: Air Quality Measurement and Effects of Pollution
- 3: Air Pollution Control Priorities and Methods
- 4: Water Quality Measurement
- 5: Raw Water Processing and Wastewater Treatment
- 6: Natural and Derived Sodium and Potassium Salts
- 7: Industrial Bases by Chemical Routes
- 8: Electrolytic Sodium Hydroxide, Chlorine, and Related Commodities
- 9: Sulfur and Sulfuric Acid
- 10: Phosphorus and Phosphoric Acid
- 11: Ammonia, Nitric Acid and Their Derivatives
- 12: Aluminum and Compounds
- 13: Ore Enrichment and Smelting of Copper
- 14: Production of Iron and Steel
- 15: Production of Pulp and Paper
- 16: Fermentation and other Microbiological Processes
- 17: Petroleum Production and Transport
- 18: Petroleum Refining
- 19: Petrochemicals
- 20: Condensation (Step-Growth) Polymer Theory
- 21: Commercial Polycondensation (Step-Growth) Polymers
- 22: Addition (Chain Reaction) Polymer Theory
- 23: Commercial Addition (Vinyl-Type) Polymers
- 1: Information Related to Soil Pollution Topics
- 2: Relevant Technical Websites by Topic
- 3: Constants, SI Units, and Multiples and Formulas
- 4: Conversion Factors, Viscosity Data
- Index