
Therapeutic Antibody Engineering
Current and Future Advances Driving the Strongest Growth Area in the Pharmaceutical Industry
- 696 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Therapeutic Antibody Engineering
Current and Future Advances Driving the Strongest Growth Area in the Pharmaceutical Industry
About this book
The field of antibody engineering has become a vital and integral part of making new, improved next generation therapeutic monoclonal antibodies, of which there are currently more than 300 in clinical trials across several therapeutic areas. Therapeutic antibody engineering examines all aspects of engineering monoclonal antibodies and analyses the effect that various genetic engineering approaches will have on future candidates. Chapters in the first part of the book provide an introduction to monoclonal antibodies, their discovery and development and the fundamental technologies used in their production. Following chapters cover a number of specific issues relating to different aspects of antibody engineering, including variable chain engineering, targets and mechanisms of action, classes of antibody and the use of antibody fragments, among many other topics. The last part of the book examines development issues, the interaction of human IgGs with non-human systems, and cell line development, before a conclusion looking at future issues affecting the field of therapeutic antibody engineering.- Goes beyond the standard engineering issues covered by most books and delves into structure-function relationships- Integration of knowledge across all areas of antibody engineering, development, and marketing- Discusses how current and future genetic engineering of cell lines will pave the way for much higher productivity
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Introduction to biologics and monoclonal antibodies
Abstract:
1.1 Introduction
1.2 Definitions of biologies
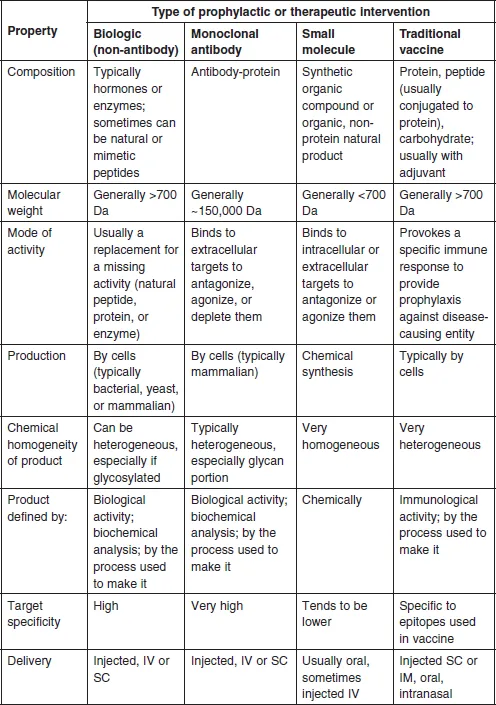
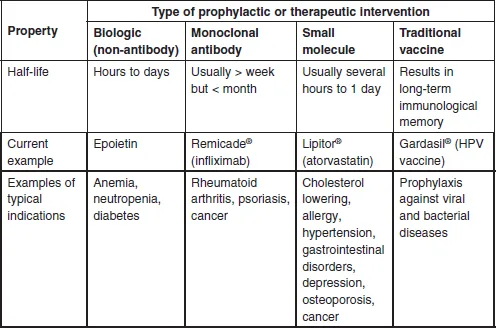
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- List of figures
- List of tables
- List of acronyms, abbreviations, and definitions
- Foreword
- Preface
- About the authors
- Chapter 1: Introduction to biologics and monoclonal antibodies
- Chapter 2: Value proposition for therapeutic monoclonal antibodies and Fc fusion proteins
- Chapter 3: Antibody structure–function relationships
- Chapter 4: Fundamental technologies for antibody engineering
- Chapter 5: Sources of antibody variable chains
- Chapter 6: Variable chain engineering – humanization and optimization approaches
- Chapter 7: Antibody interactions with the immune system
- Chapter 8: Monoclonal antibody targets and mechanisms of action
- Chapter 9: Therapeutic antibody classes
- Chapter 10: Antibody Fc engineering for optimal antibody performance
- Chapter 11: IgG glycans and glyco-engineering
- Chapter 12: Antibody fragments as therapeutics
- Chapter 13: Multiple antibody and multi-specificity approaches
- Chapter 14: FcFPs and similar constructs using Fc
- Chapter 15: Antibody-drug conjugates
- Chapter 16: Development issues: antibody stability, developability, immunogenicity, and comparability
- Chapter 17: Interactions of human IgGs with non-human systems
- Chapter 18: Cell line development
- Chapter 19: Issues facing therapeutic monoclonal antibodiesfor the future
- Useful public websites related to antibody engineering
- References
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app