
- 824 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Effect of Temperature and other Factors on Plastics and Elastomers
About this book
This book is an update to the first edition compiled and published in 1990 by William Woishnis. A lot has changed in the field since 1990 and a lot has not changed. There are new plastic materials. There has been a huge turnover in ownership of plastics producing companies. There has been a lot of consolidation, which of course means discontinued products. Thus, this update is much more extensive than the usual "next edition."It has been reorganized from a chemistry point of view. Plastics of similar polymer types are grouped into nine chapters. Each of these chapters includes an introduction with a brief explanation of the chemistry of the polymers used in the plastics.An extensive first chapter has been added as an introduction that summarizes the chemistry of making polymers, the formulation of plastics, testing and test methods, and plastic selection.Most plastic products and parts are expected to be used in environments other than room temperature and standard humidity conditions. Chapters 2-10 are a databank that serves as an evaluation of plastics as they are exposed to varying operating conditions at different temperatures, humidity, and other factors. Over 900 graphs for more than 45 generic families of plastics are contained in these chapters.Chapter 11 contains extensive mechanical and electrical data in tabular form. The tables contain data on several thousand plastics. Similarly, Chapter 12 contains thermal data on several thousand plastics. Data from the first edition have only been removed if those products were discontinued, and many products were. Product names and manufacturers have been updated.
- Detailed introductions of plastics properties, testing procedures, and principles of plastics design
- The only "databook" available on the effects of temperature and humidity conditions on plastics and elastomers
- More than 1,000 graphs and tables allow for easy comparison between products
- Covers more than 70 types of plastics, and summarizes the chemistry of each type
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Effect of Temperature and other Factors on Plastics and Elastomers by Laurence W. McKeen in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Condensed Matter. We have over one million books available in our catalogue for you to explore.
Information
1
Introduction to Plastics and Elastomers
1.1 Plastics and Polymers
The most basic component of plastic and elastomer materials is polymers. The word polymer is derived from the Greek term for “many parts.” Polymers are large molecules comprising many repeat units called monomers that are chemically bonded into long chains. Since World War II, the chemical industry has developed a large quantity of synthetic polymers to satisfy the need for a diverse range of products, including paints, coatings, fibers, films, elastomers, and structural plastics. Literally thousands of materials can be called “plastics,” although the term today is typically reserved for polymeric materials, excluding fibers, which can be molded or formed into solid or semisolid objects. As of the beginning of 2007, IDES The Plastics Web® (http://www.ides.com) listed over 65,900 different grades of plastic from over 560 suppliers.
1.1.1 Polymerization
Polymerization is the process of chemically bonding monomer building blocks to form large molecules. Commercial polymer molecules are usually thousands of repeat units long. Polymerization can proceed by one of several methods. The two most common methods are addition and condensation polymerization.
In addition polymerization, a chain reaction adds new monomer units to the growing polymer molecule one at a time through double or triple bonds in the monomer. Each new monomer unit creates an active site for the next attachment. The net result is shown in Fig. 1.1. Many of the plastics discussed in later chapters of this book are formed in this manner. Some of the plastics made by addition polymerization include polyethylene, polyvinyl chloride (PVC), acrylics, polystyrene, and polyoxymethylene (acetal).

Figure 1.1 Addition polymerization.
The other common method is condensation polymerization in which the reaction between monomer units and the growing polymer chain-end group releases a small molecule, often water as shown in Fig. 1.2. This reversible reaction will reach equilibrium and halt unless this small molecular by-product is removed. Polyesters and polyamides are among the plastics made by this process.

Figure 1.2 Condensation polymerization.
Understanding the polymerization process used to make a particular plastic provides insight into the nature of the plastic. For example, plastics made via condensation polymerization, in which water is released, can degrade when exposed to water at high temperature. Polyesters such as polyethylene terephthalate (PET) can degrade by a process called hydrolysis when exposed to acidic, basic, or even some neutral environments severing the polymer chains. As a result the polymer’s properties are degraded.
1.1.2 Copolymers
A copolymer is a polymer formed when two (or more) different types of monomers are linked in the same polymer chain, as opposed to a homopolymer where only one monomer is used. If exactly three monomers are used, it is called a terpolymer.
Monomers are only occasionally symmetric; the molecular arrangement is the same regardless of which end of the monomer molecule you are looking at. The arrangement of the monomers in a copolymer can be head-to-tail, head-to-head, or tail-to-tail. Since a copolymer consists of at least two types of repeating units, copolymers can be classified based on how these units are arranged along the chain. These classifications include:
• Alternating copolymer
• Random copolymer (statistical copolymer)
• Block copolymer
• Graft copolymer
When the two monomers are arranged in an alternating fashion, the polymer is called an alternating copolymer:

Alternating Copolymer
In the following examples A and B are different monomers that do not have to be present in a one-to-one ratio. In a random copolymer, the two monomers may follow in any order
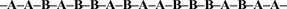
Random Copolymer
In a block copolymer, all monomers of one type are grouped together and all monomers of the other type are grouped together. A block copolymer can be ...
Table of contents
- Cover
- Title page
- Table of Contents
- Copyright
- Dedication
- Preface
- Acknowledgments
- Chapter 1: Introduction to Plastics and Elastomers
- Chapter 2: Styrenic Plastics
- Chapter 3: Polyether Plastics
- Chapter 4: Polyesters
- Chapter 5: Polyimides
- Chapter 6: Polyamides (Nylons)
- Chapter 7: Polyolefins and Acrylics
- Chapter 8: Thermoplastic Elastomers
- Chapter 9: Fluoropolymers
- Chapter 10: High Temperature Polymers
- Chapter 11: Tables of Selected ISO 10350 Properties
- Chapter 12: Tables of Selected Thermal Properties
- Appendix 1: Abbreviations
- Appendix 2: Trade Names
- Appendix 3: Unit Conversion Tables
- INDEX