
- 534 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Solid-Liquid Two Phase Flow
About this book
This book is an undertaking of a pioneering work of uniting three vast fields of interfacial phenomena, rheology and fluid mechanics within the framework of solid-liquid two phase flow. No wonder, much finer books will be written in the future as the visionary aims of many nations in combining molecular chemistry, biology, transport and interfacial phenomena for the fundamental understanding of processes and capabilities of new materials will be achieved. Solid-liquid systems where solid particles with a wide range of physical properties, sizes ranging from nano- to macro- scale and concentrations varying from very dilute to highly concentrated, are suspended in liquids of different rheological behavior flowing in various regimes are taken up in this book. Interactions among solid particles in molecular scale are extended to aggregations in the macro scale and related to settling, flow and rheological behavior of the suspensions in a coherent, sequential manner. The classical concept of solid particles is extended to include nanoparticles, colloids, microorganisms and cellular materials. The flow of these systems is investigated under pressure, electrical, magnetic and chemical driving forces in channels ranging from macro-scale pipes to micro channels. Complementary separation and mixing processes are also taken under consideration with micro- and macro-scale counterparts.- Up-to-date including emerging technologies- Coherent, sequential approach- Wide scope: microorganisms, nanoparticles, polymer solutions, minerals, wastewater sludge, etc- All flow conditions, settling and non-settling particles, non-Newtonian flow, etc- Processes accompanying conveying in channels, such as sedimentation, separation, mixing
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
The Particulate Phase: A Voyage from the Molecule to the Granule
Publisher Summary
1.1 MOLECULAR INTERACTIONS
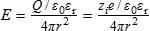
Table of contents
- Cover image
- Title page
- Table of Contents
- Preface
- List of Contributors
- Chapter 1: The Particulate Phase: A Voyage from the Molecule to the Granule
- Chapter 2: Non-Newtonian Behavior of Solid–Liquid Suspensions
- Chapter 3: Concentrated Suspensions
- Chapter 4: Motion of Particles in Fluids
- Chapter 5: Modeling the Flow of Settling Suspensions
- Chapter 6: Flow of Settling Slurries
- Chapter 7: Mixing in Solid–Liquid Systems
- Chapter 8: Classification and Separation of Solid–Liquid Systems
- Appendix A: Mathematical Operations
- Appendix B: Population Balances
- Appendix C: Tables for Use in Plug Flow in an Annulus
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app