
- 262 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This volume provides an overview of polymer characterization test methods. The methods and instrumentation described represent modern analytical techniques useful to researchers, product development specialists, and quality control experts in polymer synthesis and manufacturing. Engineers, polymer scientists and technicians will find this volume useful in selecting approaches and techniques applicable to characterizing molecular, compositional, rheological, and thermodynamic properties of elastomers and plastics.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
CHROMATOGRAPHIC TECHNIQUES
CHROMATOGRAPHY FOR ANALYTICAL ANALYSES
Chromatography may be defined as the separation of molecular mixtures by distribution between two or more phases, one phase being essentially two-dimensional (a surface) and the remaining phase, or being a bulk phase brought into contact in a counter-current fashion with the two-dimensional phase. Various types of physical states of chromatography are possible, depending on the phases involved.
Chromatography is divided into two main branches. One branch is gas chromatography, the other is liquid chromatography. Liquid chromatography can be further subdivided as shown in Figure 1.
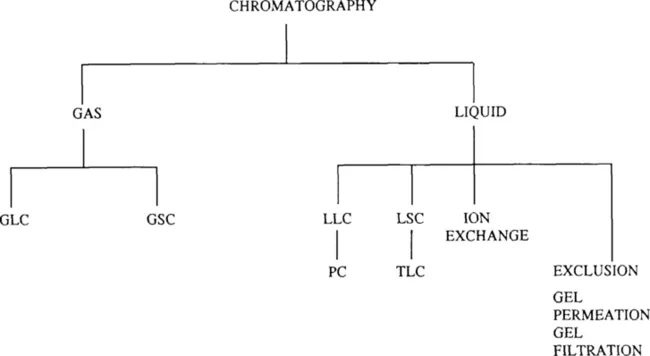
Figure 1. Shows types of chromatographic operations.
The sequence of chromatographic separation is as follows: A sample is placed at the top of a column where its components are sorbed and desorbed by a carrier. This partitioning process occurs repeatedly as the sample moves towards the outlet of the column. Each solute travels at its own rate through the column, consequently, a band representing each solute will form on the column. A detector attached to the column’s outlet responds to each band. The output of detector response versus time is called a chromatogram. The time of emergence identifies the component, and the peak area defines its concentration, based on calibration with known compounds.
GAS CHROMATOGRAPHY
General
If the moving phase is a gas, then the technique is called gas chromatography (GC). In gas chromatography the sample is usually injected at high temperature to ensure vaporization. Obviously, only materials volatile at this temperature can be analyzed.
Types of GC
If the stationary phase is a solid, the technique is referred to as gas-solid chromatography. The separation mechanism is principally one of adsorption. Those components more strongly adsorbed are held up longer than those which are not.
If the stationary phase is a liquid, the technique is referred to as gas-liquid chromatography and the separation mechanisms is principally one of partition (solubilization of the liquid phase).
Gas chromatography has developed into one of the most powerful analytical tools available to the organic chemist. The technique allows separation of extremely small quantities of material (10−6 grams).
The characterization and quantitation of complex mixtures can be accomplished with this process. The introduction of long columns, both megabore and capillary, produces a greater number of theoretical plates increasing the efficiency of separation beyond that of any other available technique. The technique is applicable over a wide range of temperatures (-40-350°C) making it possible to chromatograph materials covering a wide range of volatiles. The laboratory uses packed columns along with megabore and capillary. In this way the broadest range of chromatographic problems can be addressed.
The detector used to sense and quantify the effluent provides the specificity and sensitivity for the analytical procedure. Table 1 summarizes significant detector characteristics.
TABLE 1
SUMMARY OF DETECTOR CHARACTERISTICS
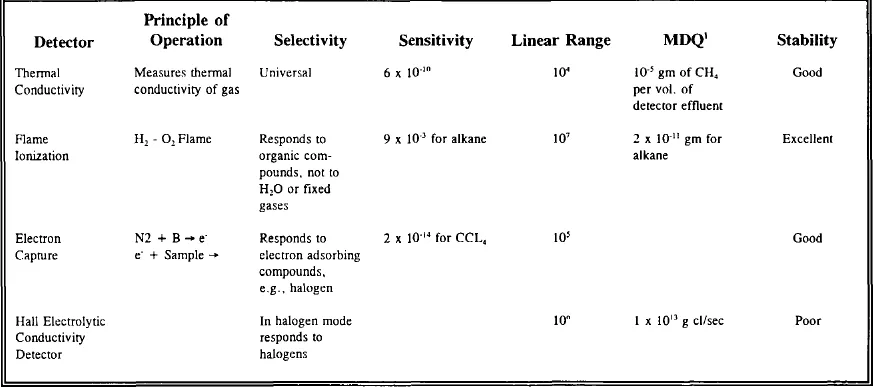
1Minimum detectable quantity.
LIQUID CHROMATOGRAPHY
General
If the moving phase is a liquid, then the technique is called liquid chromatography (LC). In liquid chromatography the sample is first dissolved in the moving phase and injected at ambient temperature. Thus there is no volatility requirement for samples. However, the sample must dissolve in the moving phase. Note that LC has an important advantage over GC: The solubility requirement can usually be met by changing the moving phase. The volatility requirement is not so easily overcome.
Types of LC
There are four kinds of liquid chromatography, depending on the nature of the stationary phase and the separation mechanism:
• Liquid/Liquid Chromatography (LLC)–is partition chromatography or solution chromatography. The sample is retained by partitioning between mobile liquid and stationary liquid. The mobile liquid cannot be a solvent for the stationary liquid. As a subgroup of liquid/liquid chromatography there is paper chromatography.
• Liquid/Solid Chromatography (LSC)–is adsorption chromatography. Adsorbents such as alumina and silica gel are packed in a column and the sample components are displaced by a mobile phase. Thin layer chromatography and most open column chromatography are considered liquid/solid chromatography.
• Ion-Exchange Chromatography–employs zeolites and synthetic organic and inorganic resins to perform chromatographic separation by an exchange of ions between the sample and the resins. Compounds which have ions with different affinities for the resin can be separated.
• Exclusion Chromatography–is another form of liquid chromatography. In the process a uniform nonionic gel is used to separate materials according to their molecular size. The small molecules get into the polymer network and are retarded, whereas larger molecules cannot enter the polymer network and will be swept our of the column. The elution order is the largest molecules first, medium next and the smallest sized molecules last. The term “gel permeation chromatography” has been coined for separations polymers which swell in organic solvent.
The trend in liquid chromatography has tended to move away from open column toward what is called high pressure liquid chromatography (HPLC) for analytical as well as preparative work. The change in technique is due to the development of high sensitivity, low dead volume detectors. The result is high resolution, high speed, and better sensitivity liquid chromatography.
Typ...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- ABOUT THE AUTHOR
- PREFACE
- NOTICE
- Chapter 1: CHROMATOGRAPHIC TECHNIQUES
- Chapter 2: THERMAL ANALYSIS
- Chapter 3: MICROSCOPY FOR POLYMER CHARACTERIZATION
- Chapter 4: ELEMENTAL AND STRUCTURAL CHARACTERIZATION TESTS
- Chapter 5: RHEOMETRY
- Chapter 6: CHEMICAL ANALYSIS OF POLYMERS
- ABBREVIATIONS OF POLYMERS
- GLOSSARY OF POLYMERS AND TESTING
- PROFESSIONAL AND TESTING ORGANIZATIONS
- GLOSSARY OF ENGINEERING AND MATERIALS TERMS
- INDEX
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Polymer Characterization by Nicholas P. Cheremisinoff in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Materials Science. We have over one million books available in our catalogue for you to explore.