![]()
Part I
State of Play and Review of Major Cooperation Initiatives
Abstract
The basic principle of international cooperation is to establish bilateral and multilateral efforts to leverage the human, scientific and financial resources and the knowledge and experience of other key regulatory authorities to avoid duplication of efforts, to make activities more efficient and to allow the focussing of limited resources on higher-risk areas of concern. This increased cooperation between worldwide regulators has necessitated proactive deliberate efforts towards convergence/harmonisation of regulation, practices and requirements to eliminate or reduce differences.
Cooperation and harmonisation of standards in the pharmaceutical domain are already a reality and have become increasingly important during recent decades, with a high level of commitment to these activities by all stakeholders. The worldwide Drug Regulatory Authorities (DRAs) have been working to end an isolationist attitude that cannot resolve current worldwide issues and challenges caused by an ever increasing globalisation. As a result, many cooperation and harmonisation initiatives have been established at the bilateral, regional and global levels as a response to the changing geo-economic-political situation.
The spectrum of collaboration varies from simple informal technical cooperation to full integration of systems and regulations. Indeed, all these initiatives can be very different in scope (some are part of a broader harmonisation initiative), level of harmonisation (depending on the political support/commitment), organisation (well-structured versus simple discussion) or advancement (established process vs. pilot projects), but they all work towards convergence of requirements and/or practices. All these multiple worldwide cooperation and harmonisation programmes have evolved rapidly over the past decades.
This book section provides the current status of this complex and broad phenomenon of cooperation, convergence and harmonisation in the pharmaceutical sector. It reviews all major global, regional and bilateral cooperation initiatives.
Keywords
Regulation; Medicine; Cooperation; Convergence; Harmonisation; Agreement; International; Regional; Bilateral; Network; Pharmaceutical; Standard; Requirement; Best Practice; European Union; WHO; ICH; ASEAN; PANDRH; SADC; GCC; APEC
There are topics that are global in nature for which resolution of related issues and problems cannot be restricted to any one country or region. These topics require cooperation between countries and harmonization of standards. Climate change, pollution, and water supply are good examples of issues that need to be resolved at a global level as they extend across borders. Public health, which includes availability of relevant medicines, is another topic that needs to be considered at a global level, as demonstrated by recent pandemic crises.
Many aspects of increased globalization also have profound implications on pharmaceutical regulation worldwide. In general, globalization of the economy (with increased travel of people and exchange of goods, finance, and information), and also globalization of the pharmaceutical market (including development, manufacture, and distribution activities), requires increased cooperation and harmonization of pharmaceutical standards and regulation. Pharmaceutical industries have asked for better harmonization of requirements for the development and manufacture of pharmaceutical products to avoid duplication of work that ultimately creates delays in drug availability [23].
In this context, harmonization of pharmaceutical regulations has naturally become an important topic of discussion among worldwide Drug Regulatory Authorities (DRAs). Over the past several decades, they have been working to end an isolationist attitude that cannot resolve current worldwide issues and challenges. As a result, many cooperative initiatives (bilateral, regional, and global) were established, and harmonization efforts have been enhanced. All these initiatives can be very different in scope (some are part of a broader harmonization initiative), level of harmonization (depending on the political support/commitment), organization (well structured versus simple discussion), or advancement (established process versus pilot projects), but they all work towards harmonization of requirements and/or practices. Increased exchange of information on a regular basis (e.g., more than 26 countries and international organizations from Australia to Vietnam now have agreements to share information with the United States Food and Drug Administration [US FDA]) [24] also contributes to the natural convergence of requirements and practices.
Harmonization models can be distinguished by their scope and objectives. Indeed, the spectrum of collaborations varies from simple technical cooperation to full integration of systems and regulations:
Simple technical and scientific intergovernmental cooperation model: In this type of cooperation model, countries agree to exchange expertise and experience to accelerate the availability of medicines (e.g., the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use [ICH], the Pan American Network for Drug Regulatory Harmonization [PANDRH], the European Union [EU]/United States of America [US] bilateral agreement).
Integration model: In this type of agreement, most of the time driven by political decision, deeper harmonization of regulation is achieved with the creation of supranational central authorities in order to support integration and/or creation of a single market (e.g., EU, the Association of Southeast Asian Nations [ASEAN]). In this case, harmonization of standards and regulations is critical in reducing trade barriers. In this model, countries give up some of their autonomy on certain matters by transferring the power to make decisions to the common supranational authority or by automatically recognizing decisions from the other party (via mutual agreement mechanisms).
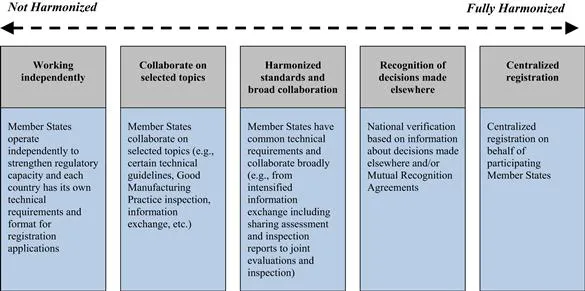
The African Medicines Registration Harmonization (AMRH) initiative has defined five identifiable levels of harmonization (Figure 1).
FIGURE 1 Levels of Harmonization. Source: New Partnership for Africa's Development (NEPAD) and WHO, “African Medicines Registration Harmonization (AMRH) Initiative: Summary, Status and Future Plans,” November 2009.
To facilitate cooperation, a Mutual Recognition Agreement (or Arrangement) (MRA) can be signed by one or more parties to mutually recognize or accept some or all aspects of one another’s requirements. They can be concluded at the technical level (e.g., the confidentiality arrangements between the US FDA and European Medicines Agency [EMA], or the MRA between EU and Australia) or at the government level (e.g., European Treaty).
I-1) Global Initiatives
These multilateral initiatives are major projects as they involve multiple organizations and countries and represent the highest degree of harmonization. The objective of this technical and scientific intergovernmental cooperation is to globally discuss scientific issues that support the decisions made by individual governments and international regulatory bodies in order to achieve global scientific consensus. The goal is to facilitate the development of new medicines and to make them available to the maximum number of people worldwide. There is no intent of full integration of systems and regulations. The main difficulty faced by these initiatives is the complexity and management of the structure due to the important number of participants (e.g., the World Health Organization [WHO] has 194 Member States) and the diversity of needs, challenges, and level of development of its members.
I-1.1) World Health Organization
The World Health Organization (WHO) was established in 1948 as a specialized agency of the United Nations (UN) [25]. It is accountable to its Member States and works closely with other entities of the UN system.
This agency has a very broad scope of responsibilities as it is the directing and coordinating authority for international health matters and public health within the UN system. WHO is well known for some of its work (e.g., the coordination of influenza surveillance and monitoring activities, emergency assistance to people affected by disasters, mass immunization campaigns or actions against Human Immunodeficiency Virus/Acquired Immunodeficiency Syndrome [HIV/AIDS], tuberculosis, and malaria). However, WHO undertakes many more activities because it is responsible for providing leadership on global health matters, shaping the health research agenda, setting norms and standards, articulating evidence-based policy options, providing technical support to countries, and monitoring and assessing health trends. Most of these core functions, as further defined in its “11th General Programme of Work,” [26] rely on cooperation and harmonization of standards.
This focus on regional and global collaboration, and especially aid from developed countries to developing countries, is aligned with the UN Millennium Development Goals (MDGs).a Indeed, the objective of these MDGs is that countries and development partners work together to improve the global situation and resolve major issues. A number of specific targets and indicators have been identified to monitor progress towards the goals. Goal 8 (“Develop a global partnership for development”) specifically recognizes the role of developed nations and addresses global cooperation and partnerships.
WHO has worked in the area of pharmaceuticals since its creation approximately 60 years ago. During this time, many products and services have been created that are widely recognized as core functions of WHO.
The role of WHO in pharmaceutical regulations is based on its constitutional mandate and various World Health Assembly (WHA) resolutions. This support is twofold. One aspect relates to the development of internationally recognized norms, standards, and guidelines. The second relates to providing guidance, technical assistance, and training in order to enable countries to implement global guidelines to meet their specific medicines regulatory environment and needs [27].
I-1.1.1) Membership
All countries that are members of the UN may become members of WHO by accepting its constitution. Other cou...