
- 780 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Cleaning with Solvents: Science and Technology
About this book
High-precision cleaning is required across a wide range of sectors, including aerospace, defense, medical device manufacturing, pharmaceutical processing, semiconductor/electronics, etc.Cleaning parts and surfaces with solvents is simple, effective and low-cost. Although health and safety and environmental concerns come into play with the use of solvents, this book explores how safe and compliant solvent-based cleaning techniques can be implemented. A key to this is the selection of the right solvent. The author also examines a range of newer "green" solvent cleaning options.This book supplies scientific fundamentals and practical guidance supported by real-world examples. Durkee explains the three principal methods of solvent selection: matching of solubility parameters, reduction of potential for smog formation, and matching of physical properties. He also provides guidance on the safe use of aerosols, wipe-cleaning techniques, solvent stabilization, economics, and many other topics.A compendium of blend rules is included, covering the physical, chemical, and environmental properties of solvents.- Three methods explained in detail for substitution of suitable solvents for those unsuitable for any reason: toxic solvents don't have to be tolerated; this volume explains how to do better- Enables users to make informed judgments about their selection of cleaning solvents for specific applications, including solvent replacement decisions- Explains how to plan and implement solvent cleaning systems that are effective, economical and compliant with regulations
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Relationship of Solvent Properties to Structure
Abstract
Keywords
1.1 Background
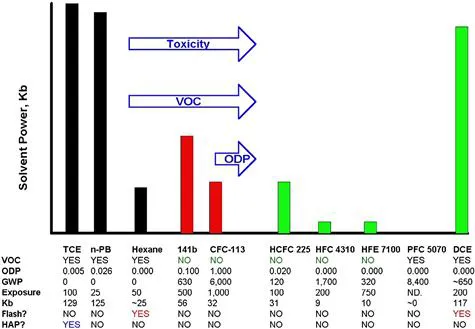
1.2 The Elements of Cleaning Solvents
This constraint eliminates metallic elements, those of substantial molecular weight, and those of low atomic weight. With some exceptions, the compounds of which these elements are constituents can’t have a molecular weight outside of the range 50 to 200.
In other words, the solvent must be similar to the soil. Since most soils are oils or greases based on carbon and hydrogen, the solvents used to clean them are too. This also means that elements normally considered inert, such as argon and helium, are not useful as components of cleaning solvents.
This constraint eliminates radioactive elements, thermally or chemically unstable elements, those of high reactivity with other elements, and those capable of producing harm to humans or the environment through their action or mere presence2.
Elemental reactivity and radioactivity again limit solvent composition. Furthermore, the solvent and its potential products of decomposition can’t be restricted by being specifically listed in environmental codes.
Since the volume of solvents used for industrial cleaning is dwarfed by the volume of solvents used as reactants, refrigerants, diluents, or inert ingredients, there must be several additional and substantial non-cleaning uses for any proposed cleaning solvent. No manufacturer will produce a solvent for use only in cleaning operations. Furthermore, net cost-in-use, from purchase to disposal, must not exceed a few dollars per pound, which mitigates against the use of elements rarely in use.
This author believes that the cost of developing new chemicals as solvents will act as a brake on the development of new solvents3. A significant part of that development cost is toxicological and environmental testing.
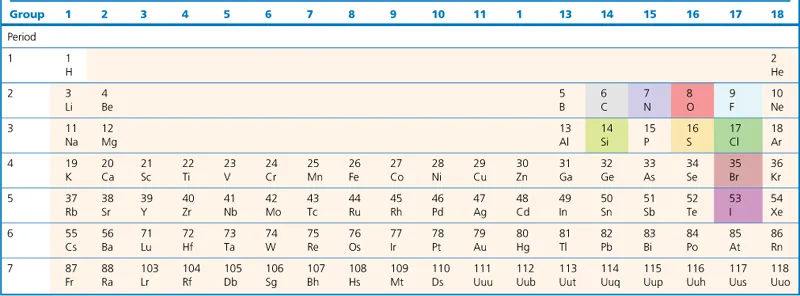
1.3 The Incredible Shrinking Periodic Table
1.4 A Solvent can be Elements Arranged in a Structure
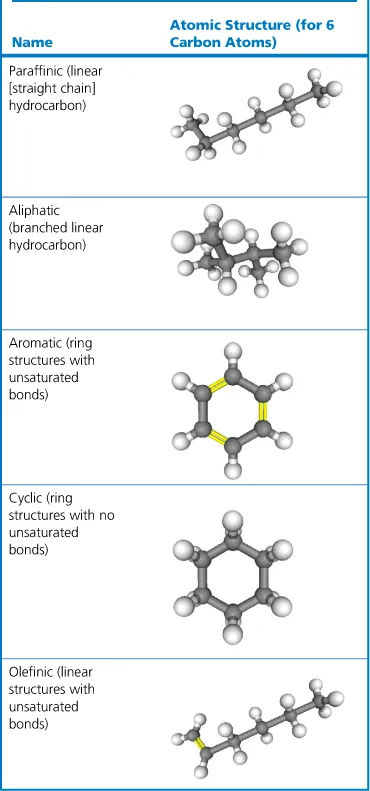
1.5 A Solvent can also be a Structure Populated with Additional Elements...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- Acknowledgments
- Disclaimer
- What You Can Do with This Book
- A Note on Organization
- Units Used in This Book
- External References Cited in This Book
- Chapter 1. Relationship of Solvent Properties to Structure
- Chapter 2. Solubility Scales (Parameters)
- Chapter 3. Solvent Selection for Specific Tasks
- Chapter 4. SHE Management (Solvent Substitution)
- Chapter 5. Toxicology of Cleaning Solvents
- Chapter 6. The VOC Exemption Game
- Chapter 7. Economics of Solvent Use
- Chapter 8. Solvent Azeotropes
- Chapter 9. Wipe Cleaning with Solvents
- Chapter 10. Cleaning with Solvent Aerosols
- Chapter 11. Stabilization of Solvents
- Chapter 12. Solvent Cleaning: Questions and Answers
- Group A. Basic Information
- Appendix A1. Basic Data about Cleaning Solvents
- Appendix A2. Estimation of Properties of Solvent Blends
- Appendix A3. Derivation of Blend Rule for Solubility Parameters
- Appendix A4. Compatibility of Wipe Cleaning Solvents with Surface Materials and Protective Gloves (With Database)
- Appendix A5. Management of Flow of Cleaning Solvents to Wet Surfaces (The Wettability Index and the Dimensionless Ohnesorge Number)
- Group B. Reduction of Ozone Formation by VOCs
- Appendix B1. Chemistry of Atmospheric Reactions of VOCs Leading to Smog
- Appendix B2. Calculation of MIR through Group Contribution Methods
- Group C. Solubility Parameters
- Appendix C1. Optimization Method for Determination of Solubility Parameters
- Appendix C2. Estimation of Hansen Solubility Parameters (HSP) from Binary Data—PES
- Appendix C3. Estimation of HSP from Multilevel Data—Bitumen
- Appendix C4. Estimation of HSP from Solvent Mixtures
- Appendix C5. Estimation of HSP from Correlations
- Appendix C6. Estimation of HSP using the “Pythagorean Theorem”
- Appendix C7. Estimation of HSP from an Equation of State
- Appendix C8. Estimation of HSP from Group Contribution Methods
- Appendix C9. Estimation of HSP for Soil Mixtures
- Appendix C10. Hoy Solubility Parameters
- Appendix C11. Values of Hansen Solubility Parameters for Solvents, Soils, and Polymers
- Appendix C12. The Teas Graph
- Group D. Solvent Substitution
- Appendix D1. Examples and Methodology of Solvent Substitution
- Appendix D2. Examples of Solvent Substitution to Achieve VOC Reduction
- Index