
- 574 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Joining and Assembly of Medical Materials and Devices
About this book
As medical devices become more intricate, with an increasing number of components made from a wide range of materials, it is important that they meet stringent requirements to ensure that they are safe to be implanted and will not be rejected by the human body. Joining and assembly of medical materials and devices provides a comprehensive overview of joining techniques for a range of medical materials and applications.Part one provides an introduction to medical devices and joining methods with further specific chapters on microwelding methods in medical components and the effects of sterilization on medical materials and welded devices. Part two focuses on medical metals and includes chapters on the joining of shape memory alloys, platinum (Pt) alloys and stainless steel wires for implantable medical devices and evaluating the corrosion performance of metal medical device welds. Part three moves on to highlight the joining and assembly of medical plastics and discusses techniques including ultrasonic welding, transmission laser welding and radio frequency (RF)/dielectric welding. Finally, part four discusses the joining and assembly of biomaterial and tissue implants including metal-ceramic joining techniques for orthopaedic applications and tissue adhesives and sealants for surgical applications.Joining and assembly of medical materials and devices is a technical guide for engineers and researchers within the medical industry, professionals requiring an understanding of joining and assembly techniques in a medical setting, and academics interested in this field.- Introduces joining methods in medical applications including microwelding and considers the effects of sterilization on the resulting joints and devices- Considers the joining, assembly and corrosion performance of medical metals including shape memory alloys, platinum alloys and stainless steel wires- Considers the joining and assembly of medical plastics including multiple welding methods, bonding strategies and adhesives
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Introduction to medical materials and devices
Abstract:
1.1 Introduction
1.1.1 An ontology for medical materials
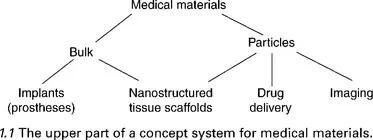
1.1.2 An ostensive definition of medical materials
| Rank | Material | N |
| 1 | Titanium | 2492 |
| 2 | Steel | 607 |
| 3 | Chromium | 391 |
| 4 | Polyethylene (PE), ultra-high molecular weight polyethylene (UHMWPE) | 389 |
| 5 | Silicone | 375 |
| 6 | Cobalt | 235 |
| 7 | Polytetrafluoroethylene (PTFE), Teflon, fluoroplastic | 220 |
| 8 | Molybdenum | 216 |
| 9 | Vitallium | 198 |
| 10 | Polypropylene (PP) | 160 |
| 11 | Polymethylmethacrylate (PMMA), acrylic | 111 |
| 12 | Polyester | 107 |
| 13 | NiTiNOL | 101 |
| 14 | Polyurethane (PU) | 72 |
| 15 | Platinum | 58 |
| 16 | Premilene (a lightweight polypropylene mesh) | 55 |
| 17 | Aluminium, alumina | 52 |
| 18 | Carbon | 51 |
| 19 | Rubber | 49 |
| 20 | Hydroxyapatite (HAp) | 45 |
| 21 | Silicon | 42 |
| 22 | Polylactic acid (PLLA) | 39 |
| 23 | Polyethyletherketone (PEEK) | 32 |
| 24 | Tantalum | 18 |
1.1.3 Surface attributes of medical materials
1.1.4 The nature of interaction
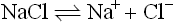
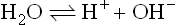
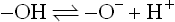
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Contributor contact details
- Woodhead Publishing Series in Biomaterials
- Part I: Fundamentals of joining and assembly in medical materials and devices
- Part II: Joining and assembly of medical metals
- Part III: Joining and assembly of medical plastics
- Part IV: Joining and assembly of biomaterial and tissue implants
- Index