
eBook - ePub
Hybrid Retrosynthesis
Organic Synthesis using Reaxys and SciFinder
- 146 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Designed to supplement existing organic textbooks, Hybrid Retrosynthesis presents a relatively simple approach to solving synthesis problems, using a small library of basic reactions along with the computer searching capabilities of Reaxys and SciFinder. This clear, concise guide reviews the essential skills needed for organic synthesis and retrosynthesis, expanding reader knowledge of the foundational principles of these techniques, whilst supporting their use via practical methodologies.
Perfect for both graduate and post-graduate students, Hybrid Retrosynthesis provides new applied skills and tools to help during their organic synthesis courses and future careers, whilst simultaneously acting as useful resource for those setting tutorial and group problems, and as a helpful go-to guide for organic chemists involved in either industry or academia.
- Ideal revision and hands on learning guide for organic synthesis
- Clearly explains the principles and practice of retrosynthesis, which is often not covered in other books
- Encourages readers to practice their synthetic knowledge supported by real life examples
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Hybrid Retrosynthesis by Michael B. Smith,John D'Angelo in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Chapter 1
Disconnections and Synthesis
This chapter briefly summarizes the theory of synthetic planning via Corey’s disconnection approach. Using simple examples, the need for a deep understanding a familiarity with organic reactions is clearly demonstrated. Finally, the necessary foundation for understanding the organization of the entire book is laid.
Keywords
Retrosynthesis; synthetic planning; disconnection; synthesis; functional group exchange; carbon-carbon bond
1.A The Disconnection Approach
To many students, the study of organic chemistry is just a grueling test of memory where they are forced to memorize countless reactions, all apparently differentiated by the most innocuous details. When asked to use those reactions to prepare new molecules, which is known as synthesis, one must have the ability to remember and use those reactions for a specific ways. One important question that students ask is, why is it necessary to know so many reactions? In fact, it is not an unfair question. The incredible diversity of structures and functional groups that appear in synthetic targets make a detailed knowledge of reactions an absolute necessity. It is probably true that an understanding of so many different reactions can only be understood by years of study, accompanied by laboratory work.
The diversity of structures leads to many synthesis problems because different carbon–carbon bonds must be made, and the incorporation of many different functional groups leads to different problems. One target molecule may contain several groups that are identical, such as a molecule with six different alcohol units, or three different ketone units. Such diversity makes synthesis virtually impossible without a thorough understanding of many different reactions, and why certain ones are better choices under specific conditions. It is therefore important to understand several different ways to make or incorporate the same functional group. On the plus side, a working knowledge of synthesis and protocols used to synthesize molecules can help with remembering reactions and also understanding them. Students in their first organic chemistry course often struggle with correlating the correct reaction and reagent with the correct functional group and may not appreciate this observation. This book attempts to fix this failure to see the forest through the trees, at least for those who have completed the two-semester organic chemistry sequence. To that end, the discussions in this book will provide a working library of common reactions and protocols used to synthesize molecules.
The chemical structure of medicines and other important molecules are characterized by the presence of many carbon atoms and often several functional groups. If such a molecule is not readily available from a commercial source, or a preparation is not found in the literature, a synthesis must be devised for that molecule. Normally, any synthesis requires choosing a commercially available molecule of fewer carbons as a starting material, and building the molecule, step-by-chemical step. Building a molecule in this manner is known as chemical synthesis, and it requires making carbon–carbon bonds to convert a smaller molecule into a larger and more complex one. Arguably, the fastest way to find out how well one understands reactions in organic chemistry is to attempt a synthesis that requires many different reactions. Planning a synthesis therefore instantly brings to light those reactions that are known and those that are not.
Traditionally, books and classroom studies provide the theoretical knowledge of organic reactions necessary to be a practicing organic chemist. Such knowledge is honed and refined in the laboratory and in the library to produce a true synthetic chemist, using knowledge of reactions, reagents, and theory to prepare organic compounds. If a planned reaction does not work, an alternative must be found, and/or research must be done to determine why the reaction did not work. All such studies require a good working understanding of organic chemistry in general, and organic reactions in particular. The power of computers and modern database searching is a great asset to the way in which modern chemists search for information in their quest to solve those synthetic problems. Indeed, the way in which chemists obtain and process knowledge has been modified and transformed by technology. In principle, this knowledge is used to modify an experiment to make it work. One can speculate on the advantage of learning reactions by computer searches. Faced with a problem in a total synthesis or any given reaction, there is no question that using computer searches to screen the chemical literature for reactions, or to find reactions for a given transformation is remarkably powerful.
This book will provide an overview of organic synthesis, and describe how to use two computer databases, REAXYS and SciFinder Scholar, to assist syntheses and find reactions. It is assumed that the reader has had at least two semesters of an undergraduate organic chemistry course before using the techniques described here.
The total synthesis of complex organic molecules demands a thorough knowledge of reactions. There are two major categories of reaction types. Reactions in one class make carbon–carbon bonds, and are called carbon–carbon bond-forming reactions. Reactions in the second class change one functional group into another, and they are called functional group exchange reactions.
Nowadays, the relationship of two molecules in a planned synthesis, say the preparation of 4 from 1, is commonly shown using a device known as a transform, defined by Nobel laureate E.J. Corey1 as: “the exact reverse of a synthetic reaction to a target structure.” The target structure is the final molecule one is attempting to prepare (in this case, alcohol 4). To begin the process, note that 4 has one more carbon atom when compared to 1. Therefore, a carbon–carbon bond must be formed as part of the synthesis. This fact also means that any analysis that will lead back to the starting material must mentally break a carbon–carbon bond in the target. Removal of fragments by mentally breaking bonds is known as a disconnection, and the overall process that leads from target back to starting material is known as retrosynthesis.
A comparison of 1 and 4 in Figure 1.1 indicates that a methyl group must be added to C2 during the course of the synthesis. In this retrosynthesis, alcohol 4 is “simplified” by mentally breaking a C–C bond that connects one methyl group to the hydroxyl (OH)-bearing carbon. This mental exercise is known as a bond disconnection. In all cases, simplification means that the disconnect products have fewer carbon atoms when compared to the target. This observation is usually determined by the fact that the starting material for a synthesis has fewer carbon atoms than the final target, and any retrosynthesis works from the target back to the starting material. Therefore, each disconnection should simplify the target if possible. Simplification can involve disconnection of a large piece or a small piece. For complex molecules, disconnection of a large fragment is almost always preferred, but for small molecules such as 4, loss of even one carbon atom constitutes simplification.
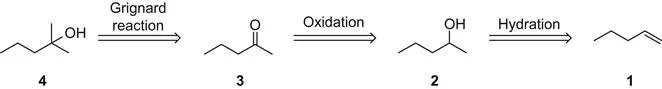
Any synthesis takes an available molecule (called the starting material) and transforms it by a series of reactions into a molecule that is required for some purpose (the target). For a molecule of any complexity, the reactions employed must include both carbon–carbon bond forming reactions and functional group transformations. The number and nature of the reactions are unknown by just looking at the target. Therefore, the purpose of the retrosynthetic analysis is to define both the number and nature of the requisite reactions for a given target.
This disconnected methyl carbon is attached to the OH-bearing carbon in 4, and familiarity with chemical reactions suggests that this bond can be made by the reaction of a methyl reagent to a carbonyl group. In fact, this disconnection was chosen because it is known to the authors that an acyl addition reaction of a Grignard reagent to ketone 3 will give 4. This knowledge arises from understanding reactions that change a ketone to an alcohol and others tha...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- Common Abbreviations
- Summary of Reactions in Chapter 8
- Chapter 1. Disconnections and Synthesis
- Chapter 2. Making Carbon–Carbon Bonds
- Chapter 3. Computer-Assisted Syntheses
- Chapter 4. A Hybrid Retrosynthesis Approach
- Chapter 5. Creative Strategies to Searching for Reagents
- Chapter 6. Stereochemistry
- Chapter 7. Molecules of Greater Complexity
- Chapter 8. Common Fundamental Reactions in Organic Chemistry
- Index