
- 644 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Microsomes, Drug Oxidations and Chemical Carcinogenesis V1
About this book
Microsomes, Drug Oxidations, and Chemical Carcinogenesis, Volume I, documents the proceedings of the 4th International Symposium on Microsomes and Drug Oxidations held in Ann Arbor, July 1979. The symposium reviewed progress in the understanding of scientific and biomedical problems from a biochemical, biophysical, pharmacological, and toxicological perspective. The book contains 117 contributions made by researchers at the symposium, which are organized into three sections. The papers in Section I focus on the chemical and physical characteristics of cytochrome P-450. Section II examines the mechanisms of action of cytochrome P-450 and related enzymes. The studies in Section III deal with the influence of membrane structure and protein synthesis on electron transfer components. This book seeks to aid future progress in understanding the complexities of metabolic transformations by these versatile enzyme systems that act on physiologically important lipids as well as on a wide array of foreign substances, including drugs, anesthetics, industrial chemicals, food additives, pesticides, carcinogens, and nonnutrient dietary chemicals.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Microsomes, Drug Oxidations and Chemical Carcinogenesis V1 by Minor Coon in PDF and/or ePUB format, as well as other popular books in Medicine & Nutrition, Dietics & Bariatrics. We have over one million books available in our catalogue for you to explore.
Information
SECTION I
CHEMICAL AND PHYSICAL CHARACTERIZATION OF CYTOCHROME P-450
ENZYMATIC ACTIVATION OF MOLECULAR OXYGEN
O. Hayaishi, O. Takikawa, M. Sono and R. Yoshida, Department of Medical Chemistry, Kyoto University Faculty of Medicine, Kyoto, Japan
Publisher Summary
This chapter discusses enzymatic activation of molecular oxygen. The chapter discusses about tryptophan 2,3-dioxygenase or pyrrolase, which catalyzes a rupture of the pyrrole ring of tryptophan, forming formylkynurenine as the reaction product. Tryptophan dioxygenase is a hemoprotein containing protoporphyrin IX as its sole prosthetic group. The native, ferric form of the enzyme exhibits a spectrum typical of a high-spin hemoprotein. It can be reduced to a ferrous form, which, in the presence of the substrate tryptophan, binds with oxygen to form a new spectral species with peaks at 418, 545, and 576 nm. This new spectral species, which can also be observed during the steady state of the reaction, represents a ternary complex of enzyme–substrate and oxygen and was established to be the obligatory intermediate of reaction by kinetic analysis using a stopped-flow apparatus. The chapter presents a comparison of monooxygenase activity of various hemoproteins. Turnover number of various hemoproteins is expressed in terms of mol p-aminophenol produced/min/mole of heme at 37°. The chapter also illustrates the dependency of the indoleamine dioxygenase catalyzed monooxygenase reaction.
Studies on the mechanisms of biological oxidation processes were initiated by the French chemist Lavoisier some two hundred years ago. He demonstrated that respiration is a slow combustion of various nutrients in the body, and that the inhaled oxygen oxidized carbon atoms of sugars and fats to carbon dioxide, CO2 and hydrogen atoms to water, H2O. Accordingly, Lavoisier defined the term “oxidation” as the addition of oxygen atoms to a substrate X, while the opposite process, that of reduction, was considered to be the removal of oxygen from an oxide, XO2 to X and O2. In the century that followed Lavoisier’s discoveries, there were many modifications and extensions of his basic ideas, and a number of theories were proposed to explain how molecular oxygen is activated and how it reacts with organic substrates.
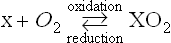
At the onset of the present century, however, the involvement of molecular oxygen per se in biological oxidation processes was vigorously challenged by Professor Heinrich Wieland, a distinguished chemist and a Nobel Laureate from Germany. In 1923, Wieland authored a book titled, “On the Mechanism of Oxidation” and proposed ‘the theory of enzymatic dehydrogenation’ as the essential principle of biological oxidation (1)
According to the dehydrogenation theory, the principle of biological oxidation processes is the removal of hydrogen atoms, or more precisely speaking, electrons, from the substrate molecule XH2, and their transfer to an appropriate acceptor A, such as coenzymes and various dyes. The opposite process, namely the addition of hydrogen atoms, was defined as “reduction”.
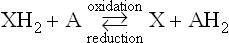
What then is the role of molecular oxygen in biological oxidation processes? Oxygen molecules may, in some instances, serve as the immediate electron acceptor as shown below.
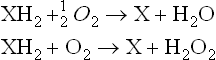
Molecular oxygen accepts hydrogen atoms and is reduced to water or hydrogen peroxide. However, it is never incorporated into the substrate as was originally proposed by Lavoisier and his contemporaries. The dehydrogenases that utilize molecular oxygen as a hydrogen acceptor are termed “oxidases”; cytochrome oxidase and D-amino acid oxidase are typical examples of these oxidases, each producing water and hydrogen peroxide from molecular oxygen, respectively. Thus, according to Professor Wieland, the direct addition of molecular oxygen to a substrate was considered completely irrelevant to biological oxidation.
In 1949, during a study of tryptophan metabolism in a bacterial species Pseudomonad, we isolated and purified a novel enzyme, pyrocatechase from the microorganism. Pyrocatechase catalyzed the oxidative cleavage of the aromatic structure of catechol, producing cis, cis-muconate as the reaction product (Fig. 1). Because of the unusual properties of this enzyme, we suspected that it might catalyze direct addition of O2 to substrate.
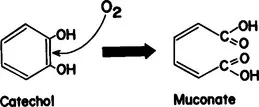
FIGURE 1 Pyrocatechase.
A set of experiments was performed in which a heavy oxygen isotope, oxygen-18 was used in air, in Experiment I, or in the form of water in Experiment II. The product, muconic acid, was isolated and analyzed for the 18O content using a mass spectrometer. Contrary to the then generally held belief, the results clearly established that the oxygen atoms incorporated into the product molecules were derived exclusively from molecular oxygen but not from the oxygen of the water molecule (2). If pyrocatechase were a dehydrogenase or an oxidase, the incorporated oxygen atoms should have been derived from water, according to the theory of Professor Wieland.
Concurrently and independently, Prof. Mason in Oregon demonstrated that phenolase of the mushroom, incorporated one atom of molecular oxygen into a substrate (3). These findings therefore established that “oxygen fixation” did indeed occur in biological systems. We therefore proposed that the enzymes responsible for these reactions be termed “dioxygenase” and “monooxygenase”, respectively. In essence, this was the revival of the old concept of Lavoisier. Since then oxygenases have been isolated from animals, plants and microorganisms and some have been highly purified and crystalized. The structure and the mechanism of action of oxygenases were also investigated in detail.
Dioxygenases are now subdivided into two classes as shown. in Table I. Typical examples of intra and intermolecular dioxygenases are pyrocatechase and α-ketoglutarate dependent dioxygenases, respectively.
TABLE I
Dioxygenas...
Table of contents
- Cover image
- Title page
- Table of Contents
- Inside Front Cover
- Copyright
- SENIOR AUTHORS FOR VOLUMES I AND II
- PREFACE
- ACKNOWLEDGMENTS
- SECTION I: CHEMICAL AND PHYSICAL CHARACTERIZATION OF CYTOCHROME P-450
- SECTION II: MECHANISM OF ACTION OF CYTOCHROME P-450 AND RELATED ENZYMES
- SECTION III: INFLUENCE OF MEMBRANE STRUCTURE AND PROTEIN SYNTHESIS ON ELECTRON TRANSFER COMPONENTS