
- 370 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Special Distillation Processes
About this book
Special distillation processes are required for separation of mixtures close to boiling point or for forming azeotrope mixtures into their pure components. In Special Distillation Processes, the authors focus on latest developments in the field, such as separation methods that may prove useful for solving problems encountered during research. Topics include extraction, membrane and adsorption distillation involving the separation principle, process design and experimental techniques. The relationship between the processes and the techniques are also presented. Comprehensive and easy-to-read, this book provides key information needed to understand the processes and is a valuable reference source for chemical engineers as well as students wishing to branch out in chemical engineering.* The only comprehensive book available on special distillation processes* Contains a thorough introduction to recent developments in the field* A valuable reference for students and engineers in chemical engineering
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Chapter 1
Thermodynamic fundamentals
This chapter presents the thermodynamic fundamentals closely related with special distillation processes, which may facilitate readers to understand the separation principle discussed in the subsequent chapters. Some novel contents, which are commonly not involved in many classic thermodynamic texts, but necessary for the understanding, such as salt effect, nonequilibrium thermodynamic analysis and multi-component mass transfer, are covered. In accordance with the step-by-step rule, the section of vapor-liquid phase equilibrium is present at the beginning, and thus there is a little overlapping with common texts.
1 VAPOR-LIQUID PHASE EQUILIBRIUM
Phase equilibrium can be sorted into vapor-liquid, liquid-liquid, vapor-liquid-liquid, etc. Vapor-liquid phase equilibrium as the most applied and calculable form of phase equilibrium plays a major role in special distillation processes, particularly with respect to energy requirements, process simulation and sizing equipment. Thus, in this chapter this content is more highlighted. But, for the sake of immiscibility, sometimes the occurrence of two-liquid phase in the distillation column is inevitable. So on the foregoing basis vapor-liquid-liquid equilibrium is also briefly mentioned in this chapter. The details about how to numerically calculate the problem on vapor-liquid-liquid equilibrium are described in chapter 3 (azeotropic distillation) where vapor-liquid-liquid equilibrium is involved.
1.1 The equilibrium ratio
Equilibrium is defined as a state that will be returned to its initial state after any short, small mechanical disturbance of external conditions. According to the knowledge of physical chemistry, within any closed system where phase equilibrium exists, the total Gibbs free energy for all phases is a minimum because at this time there is no heat and mass transfer in the system. This is the starting point for studying phase equilibrium. On the other hand, from classical thermodynamics, the total Gibbs free energy and the change of Gibbs free energy in a single phase, i-component system are:
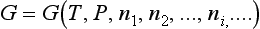
and
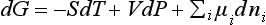
respectively, where T (temperature), P (pressure), n1, n2, · · ·, ni (mole number of components 1, 2, … i, …, etc, respectively) are independent variants.
Thus, the change...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- Chapter 1: Thermodynamic fundamentals
- Chapter 2: Extractive distillation
- Chapter 3: Azeotropic distillation
- Chapter 4: Catalytic distillation
- Chapter 5: Adsorption distillation
- Chapter 6: Membrane distillation
- Chapter 7: Pressure-swing distillation
- Chapter 8: Other distillation techniques
- Subject Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Special Distillation Processes by Zhigang Lei,Biaohua Chen,Zhongwei Ding in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.