INTRODUCTION SCOPE AND TERMINOLOGY
The term “physical adsorption” or “physisorption” refers to the phenomenon of gas molecules adhering to a surface at a pressure less than the vapor pressure. The attractions between the molecules being adsorbed and the surface are relatively weak and definitely not covalent or ionic. In Table 1 definitions used in this book and in most of the literature on physisorption are given [1].
For most adsorption experiments the temperature at which the measurements are made is less than the triple point of the gas being used but above its freezing point. This being the case, one would normally expect that the adsorbate characteristics resemble the liquid phase rather than the solid phase of the adsorptive. This is the normal assumption used for most adsorption theories. The principle measurement performed as an adsorption experiment is the measurement of the adsorption isotherm. The adsorption isotherm is the measurement of amount adsorbed versus adsorptive pressure at constant temperature. This is the easiest measurement to make. Another type of measurement is calorimetry. One form of calorimetry measures the amount of heat evolved as the adsorptive is adsorbed. Another form measures the heat capacity of the adsorbate. There are various forms of calorimetry but the most accurate methods are very difficult to perform and only a few examples are available in the literature. Another form of calorimetry, which is easier to perform, is scanning calorimetry. This calorimetry form is a good tool to determine qualitative features of the adsorption and to yield a fair indication of the physical quantities.
GENERAL DESCRIPTION OF PHYSISORPTION
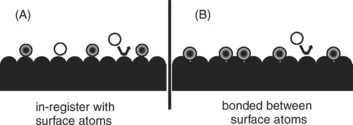
For purpose of this book distinctions will be made between physical adsorption for the liquid-like state and in the solid-like state. Figs. 1 and 2 illustrate the atomic scale difference between the two types of adsorption. For solid-like state of Fig. 1 the adsorbate molecules are located on definite sites in relation to the underlying atoms of the adsorbent. For example, they lie directly over one of the atoms or in between two or three atoms in a defined geometry. One could refer to this as an “in-register” adsorption or even “epitaxy”. Chemisorption, where the attraction between the adsorbate and adsorbent is a covalent or ionic bond, would be an example of such adsorption. Adsorption well below the triple point in temperature would also be expected to follow this pattern. Additional adsorption above the first layer, might also be “in register”.
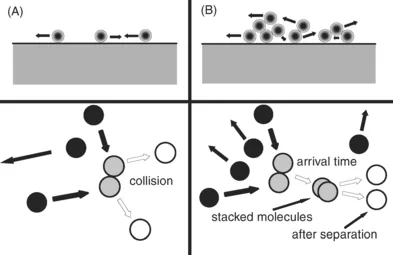
The other mode of adsorption, which is the subject of this book, is illustrated in Fig. 2, of which two theoretical sub-possibilities exists. For this adsorption, referred to as physisorption, in adsorbent provides an overall attraction for which no particular site has a strong enough attraction to localize the adsorbate. In other words, the adsorbate molecules are free to skate over the entire surface, at least for a fair distance, even though there might be bumpy spots. For this physisorption picture there can be further distinctions, one where the adsorbate is behaving as a gas and there is only adsorption on top of the adsorbate, or one where the adsorbate behaves like a liquid, where adsorbate molecules can roll over one another and an adsorptive molecule can adsorb upon an adsorbate molecule. Most adsorption isotherms are performed under conditions where the liquid-like condition is assumed to exist. Calculations of the gas-like state indicate that the amount that can be adsorbed in this fashion is very low for most practical experimental conditions. Nevertheless, one would expect some of this to exist even with the presence of the liquid-like adsorbate.
MEASURING THE SURFACE AREA BY PHYSISORPTION
There are two principal methods to measure the adsorption isotherm, volumetric and gravimetric. In both methods the adsorbent is held at a constant temperature, usually near or at the boil...