![]()
Chapter 1
Introduction to Therapeutic Drug Monitoring
Frequently and Less Frequently Monitored Drugs
Amitava Dasgupta
Department of Pathology and Laboratory Medicine, University of Texas-Houston, Houston, TX
Outline
Introduction
Drugs that Require Therapeutic Drug Monitoring
Benefits of Therapeutic Drug Monitoring
Pathophysiological Conditions and other Factors that Affect Drug Concentrations
Basic Pharmacokinetics
Genetic Variations in Drug Metabolism and Therapeutic Drug Monitoring
Effect of Gender Difference and Pregnancy on Drug Response and Metabolism
Effect of Age on Drug Response and Metabolism
Drug Disposition in Uremia
Drug Disposition in Hepatic Disease
Cardiovascular Disease and Drug Disposition
Thyroid Dysfunction and Drug Disposition
Therapeutic Drug Monitoring of Various Drug Classes
Frequently and Less Frequently Monitored Anticonvulsants
Frequently and Less Frequently Monitored Cardioactive Drugs
Frequently Monitored Anti-Asthmatic Drugs
Frequently and Less Frequently Monitored Antidepressants
Frequently Monitored Immunosuppressants
Frequently and Less Frequently Monitored Antibiotics
Less Frequently Monitored Drugs: Antiretroviral Agents
Frequently and Less Frequently Monitored Antineoplastic Drugs
Conclusions
Introduction
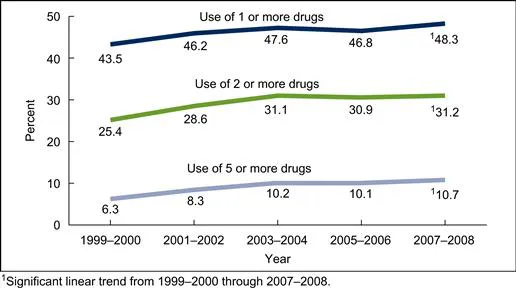
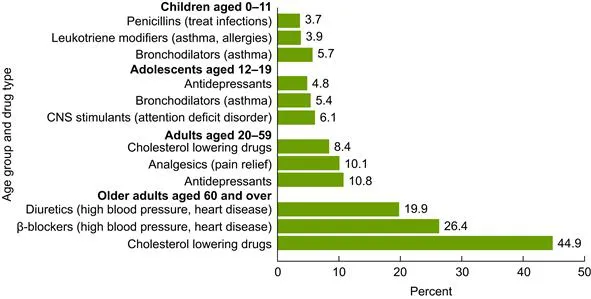
There are over 6000 prescription and non-prescription (often termed as over-the-counter) drugs approved by the FDA (Federal Drug Administration of the United States government) for clinical use. The spending for prescription drugs was US$234.1 billion in 2008, which was more than double the amount of money spent in 1999. The percentage of Americans who had used at least one prescription drug in the past month increased from 44% in 1999–2000 to 48% in 2007–2008, and, as expected, the percentage of Americans using five or more drugs also increased steadily during this period (Fig. 1.1). People aged over 60 years required more prescription drugs than younger people: only 7.9% of Americans between the ages of 20 and 59 used five or more drugs, but 36.7% of Americans aged over 60 years used five or more medications. The most commonly used drugs, based on drug use in the 2007–2008 survey, are as follows: children (age up to 11 years), bronchodilators; adolescents (12–19 years), central nervous system stimulants; adults (20–59 years), antidepressants; and elderly patients, cholesterol-lowering medications. The percentages of the most often used prescription drugs in various age groups of the American population are shown in Fig. 1.2. Diuretics and beta-blockers are also commonly used by adults and older Americans. People without a regular place for health care, health insurance, or prescription drug benefits had lower use of prescription drugs [1].
FIGURE 1.1 Trend in use of prescription drugs among Americans.
(Source: Reference 1: US Government Document).
FIGURE 1.2 Percentage of prescription drugs used most often in various age groups of American population.
(Source: Reference 1: US Government Document).
Many patients, especially the elderly, take drugs for controlling chronic conditions. Although none of the non-prescription drugs requires therapeutic drug monitoring, except for salicylate and acetaminophen in cases of overdose or attempted suicide, some prescription medications require routine therapeutic drug monitoring. Fortunately, the number of such drugs requiring routine monitoring is relatively low. Approximately 26 prescription drugs are frequently monitored in a majority of hospital-based laboratories using commercially available immunoassays and automated analyzers. In addition, approximately 25–30 drugs are subjected to therapeutic drug monitoring less frequently, and immunoassays are available for only a few such drugs. Therefore, chromatographic techniques are used for monitoring of these drugs, and such tests are only available in clinical laboratories of major medical centers and academic centers as well as reference laboratories. The most sophisticated method for therapeutic drug monitoring is liquid chromatography combined with mass spectrometry or tandem mass spectrometry (see Chapter 3 for a detailed discussion on this topic).
Therapeutic drug monitoring not only consists of measuring the concentration of a drug in a biological matrix but also involves the proper interpretation of the value using pharmacokinetic parameters, drawing appropriate conclusions regarding the drug concentration and dose adjustment. The International Association for Therapeutic Drug Monitoring and Clinical Toxicology adopted the following definition for drug monitoring [2]:
Therapeutic drug monitoring is defined as the measurement made in the laboratory of a parameter that, with appropriate interpretation, will directly influence prescribing procedures. Commonly, the measurement is in a biological matrix of a prescribed xenobiotic, but it may also be of an endogenous compound prescribed as a replacement therapy in an individual who is physiologically or pathologically deficient in that compound.
Therapeutic drug monitoring has been used in clinical practice to individualize drug therapy since the beginning of the 1970s. The goal of such monitoring is to optimize the pharmacological responses of a drug while avoiding adverse effects. Traditionally, therapeutic drug monitoring involves measuring drug concentration in a biological matrix – most commonly serum or plasma – and interpreting these concentrations in terms of relevant clinical parameters. Whole blood is the preferred matrix for therapeutic drug monitoring of immunosuppressants except for mycophenolic acid. For success of a therapeutic drug monitoring program, good communication between clinicians, laboratory professionals and pharmacists is essential. In one report, the authors clearly documented that the intervention of pharmacists significantly improved the appropriateness of therapeutic drug monitoring use and significantly reduced unnecessary costs. In addition, the authors commented that using a screening checklist including the indication for therapeutic drug monitoring, specimen collection time and data needed for proper interpretation of the drug level can improve appropriateness of utilization of the therapeutic drug monitoring service [3].
Drugs that Require Therapeutic Drug Monitoring
As stated earlier, only a small fraction of prescription drugs require therapeutic drug monitoring because for most prescription drugs there is a wider difference between therapeutic and toxic concentrations. For example, the therapeutic range of acetaminophen found in many over-the-counter drugs is 10–30 μg/mL, while toxicity is encountered at serum or plasma concentrations over 200 μg/mL. Because of the more than six-fold difference between the upper end of the therapeutic and lower end of the toxic concentration, acetaminophen is not monitored except in the case of a suspected overdose. In contrast, the therapeutic range of phenytoin is 10–20 μ/mL while toxicity may be encountered at a concentration of 30 μg/mL. Genetic polymorphism can precipitate phenytoin toxicity after a therapeutic dose. Ramasamy et al. described a case where a female developed phenytoin toxicity after administration of a therapeutic dosage of 300 mg per day of phenytoin. Her serum phenytoin level was 33 μg/mL. She was homozygous for a CYP2C9∗3∗3 mutation that led to a marked decrease in enzymatic activity of CYP2C9, which is responsible for metabolism of phenytoin [4]. Situations where therapeutic drug monitoring is beneficial include the following:
1. Where there is difficulty in interpreting therapeutic or low toxicity levels of a drug based on clinical evidence alone and there is no clearly defined clinical parameter for dose adjustment.
2. Where correlation between serum or whole blood drug concentration and dosage is poor.
3. Where there is a narrow therapeutic range, so the dose of a drug which produces the desired therapeutic concentrations in one patient may cause toxicity in another patient.
4. Where toxicity of a drug may lead to hospitalization, irreversible organ damage and even death, but an adverse drug reaction may be avoided by therapeutic drug monitoring.
5. Where there is a correlation between serum or whole blood concentration of the drug and its therapeutic response or toxicity.
6. Where there are clinical indications for therapeutic drug monitoring – for example, poor response to the drug, suspected treatment failure due to non-compliance, or signs of toxicity despite no dosage adjustment.
For strongly protein-bound drugs (protein binding > 80%), a better correlation may be observed between unbound (free) drug concentration (rather than traditionally monitored total drug concentration) and clinical outcome, because it is only the unbound drug that is responsible for the drug’s pharmacological action. For example, adjusting phenytoin dosage in patients based on their serum phenytoin concentrations rather than seizure frequencies not only decreases morbidity but also prevents phenytoin toxicity in these patients. Peterson et al. reported that, in their study involving 114 patients, free phenytoin concentrations correlated better with clinical picture than did total phenytoin concentrations [5] (see Chapter 4 for further details).
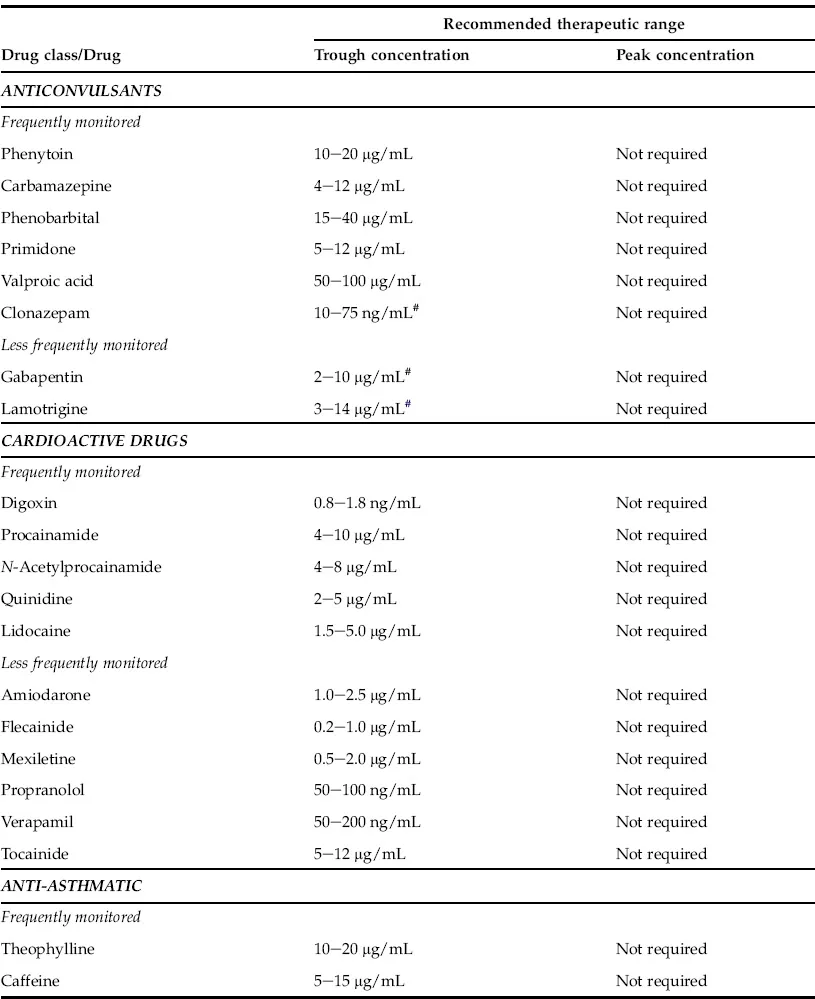
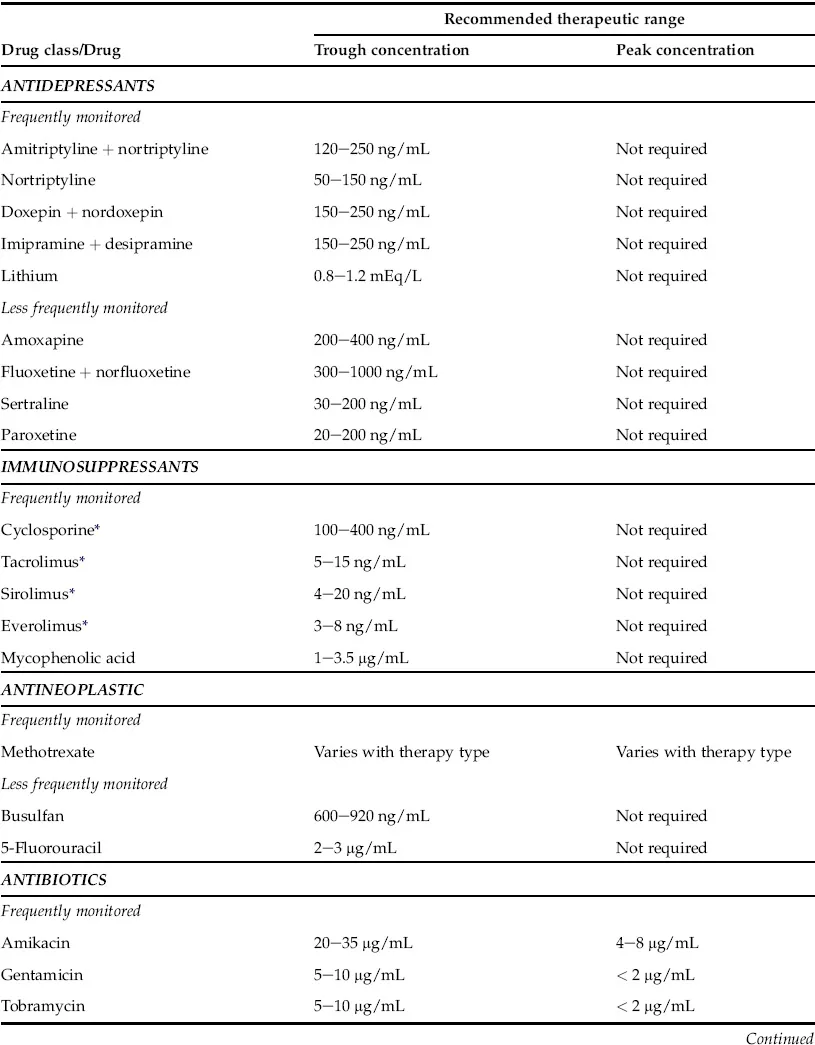
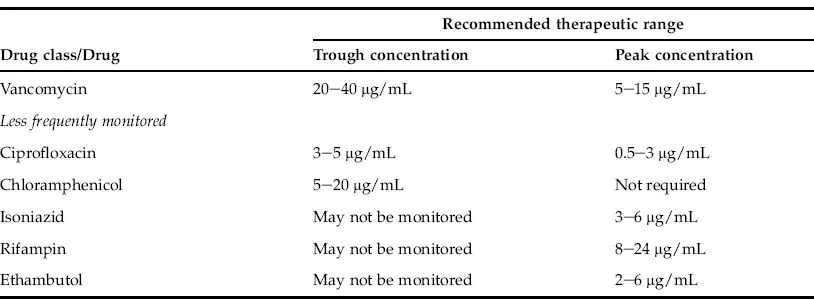
Usually, anticonvulsants, cardioactive drugs, immunosuppressants, anti-asthmatic drugs, antidepressants, antiretroviral drugs, antineoplastic drugs and antibiotics with narrow therapeutic windows, such as vancomycin and aminoglycosides, require routine therapeutic drug monitoring. In most instances the trough blood level (15–30 minutes prior to the next dosage) is the preferred specimen for therapeutic drug monitoring except for certain antibiotics (vancomycin and aminoglycosides), where both peak and trough drug levels are monitored. Vancomycin and aminoglycoside can produce serious nephrotoxicity and ototoxicity. Suggested therapeutic ranges for commonly monitored drugs as well as less commonly monitored drugs are listed in Table 1.1.
TABLE 1.1 Frequently and Less Frequently Monitored Therapeutic Drugs
Therapeutic ranges based on published literature, books as well as ranges adopted by reputed national reference laboratories such as Mayo Medical Laboratories and ARUP laboratories. However, therapeutic ranges vary widely among different patient populations and each institute should establish their own guidelines. These values are for the purpose of providing examples only.
Benefits of Therapeutic Drug Monitoring
Benefits of therapeutic drug monitoring are listed in Table 1.2. In general, many drugs are used as a prophylactic to prevent clinical symptoms – for example, phenytoin is given to a patient in order to prevent an episode of seizure. However, non-compliance has serious clinical consequences, and therapeutic drug monitoring is very useful in identifying such non-compliance. Mattson et al. commented that a zero drug level, subtherapeutic levels or variable drug levels are indicators of non-compliance in a medication where therapeutic drug monitoring is available. In the authors’ experience, 93% of their patients showe...