
- 700 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Significant Pharmaceuticals Reported in US Patents
About this book
Significant Pharmaceuticals Reported in US Patents identifies the next generation of pharmaceuticals reported in US Patents. This "hands-on" title provides explicit laboratory methods for preparing the most recent and effective medications. Each entry documents the biological testing protocols used to evaluate a drug and the significance of the current treatment agent over previous methods. Pharmaceuticals are included in this review only if at least two of the following criteria were met: Effectiveness in treating an illness, Innovative, ease of preparation, synergy with existing Medications.
Pharmaceuticals are reported for 27 separate classes of illness, including: AIDS, Alzheimer's Disease, Cardiovascular Disorders, Diabetes, Epilepsy, Hepatitis C, Osteoporosis, Obesity and Sleep Disorders.
Significant Pharmaceuticals Reported in US Patents has been designed to be used as both a reference and synthetic guide for pharmaceutical, medicinal and organic chemists and graduate students.
Researchers working in other areas will also find the information valuable as in many instances intermediates or the next generation pharmaceutical are readily convertible into other industrial products including: anti-oxidants, chemical additives, herbicides, polymer precursors, water purification agents. Clear structural depictions of reagents and chemical transformations have been supplied to permit the identification of other future applications.
- Identifies next generation pharmaceuticals
- Provides practical preparation methods for each active agent and derivatives
- Documents the analytical characterization and biological testing results of active agents
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Significant Pharmaceuticals Reported in US Patents by Thomas F. DeRosa in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Analytic Chemistry. We have over one million books available in our catalogue for you to explore.
Information
Topic
Physical SciencesSubtopic
Analytic ChemistryChapter I
Acquired Immune Deficiency Syndrome
I Antioxidants, Preparation Methods, and Uses
Title Antioxidants, Preparation Methods and Uses
J. Oiry et al., US Patent 6,989,372 (January 24, 2006)
J. Oiry et al., US Patent 6,989,372 (January 24, 2006)
Assignee Centre National de la Recherche Scientifique and Commissariat a l'Engergie Atomique
Utility Treatment of Pathologies Associated with Glutathione Depletion
Invention Significance Reactive oxygen species play an important role in human pathologies and are particularly associated with retroviral infections such as human immunodeficiency virus (HIV). Intracellular defense against oxidative species is controlled by glutathione. To contain these reactive oxygen species effects, free radical scavengers have been prepared that increase glutathione reserves.
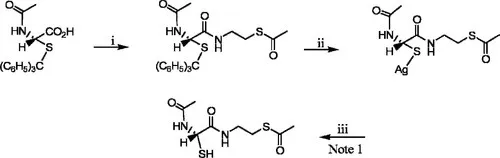
Reaction

i- N-Methylmorpholine, isobutyl chloroformate, EtOAc, S-acetylcysteamine · HCl
ii- Methyl alcohol, CHCl3, silver nitrate, pyridine
iii- CHCl3, hydrochloric acid
Experimental
1. Preparation of N-(N-acetyl-S-trityl-l-cysteinyl)-S-acetylcysteamine
A solution of N-acetyl-S-trityl-l-cysteine (0.71 mmol) and 80μ1 N-methylmorpholine dissolved in 5 ml of EtOAc was stirred at–15°C, then treated with 93μl isobutyl chloroformate. After 15 minutes, S-acetylcysteamine hydrochloride (0.71 mmol) and an additional 80μl N-methyl-morpholine were added and the mixture stirred for 15 minutes at – 15°C and then 3 hours at ambient temperature. N-Methylmorpholine hydrochloride was then filtered off and the mixture was washed twice with 2.5 ml of EtOAc and concentrated. The gummy residue was purified by flash chromatography with silica gel using EtOAc/30% petroleum ether and the product isolated in 55% yield as a colorless powder, mp=111−113°C.
Rf = 0.41, EtOAc/petroleum ether, 9:1
[α]D20=+10.5° (c. 0.8, CHCl3
1H NMR (CDCl3) δ (ppm) 1.90 (s, 3H, NCOCH3), 2.29 (s, 3H, SCOCH3), 2.48 (dd, J = 5.7, 12.9Hz, 1H, β Ha cys), 2.82 (dd, J = 6.4, 12.9Hz, 1H, β Hb cys), 2.92–3.01 (m, 2H, NCH2CH2S), 3.32–3.42 (m, 2H, NCH2CH2S), 4.07–4.20 (m, 1H, α H cys), 5.70 (d, J = 7.6Hz, 1H, NH cys), 6.34 (t, J = 5.5Hz, 1H, NHCH2), 7.19–7.35 and 7.40–7.47 (2m, 15H, aromatic H)
MS (FAB +/NBA + K +) m/z 545 (M + K)+, 507 (M + H)+; (FAB-/NRA) m/z 505 (M-H)−
Analysis Calc. for C28H30N2O3S2 (506): C, 66.40; H, 5.93; N, 5.53. Found: C, 66.17; H, 6.00; N, 5.81
A solution of N-acetyl-S-trityl-l-cysteine (0.71 mmol) and 80μ1 N-methylmorpholine dissolved in 5 ml of EtOAc was stirred at–15°C, then treated with 93μl isobutyl chloroformate. After 15 minutes, S-acetylcysteamine hydrochloride (0.71 mmol) and an additional 80μl N-methyl-morpholine were added and the mixture stirred for 15 minutes at – 15°C and then 3 hours at ambient temperature. N-Methylmorpholine hydrochloride was then filtered off and the mixture was washed twice with 2.5 ml of EtOAc and concentrated. The gummy residue was purified by flash chromatography with silica gel using EtOAc/30% petroleum ether and the product isolated in 55% yield as a colorless powder, mp=111−113°C.
Rf = 0.41, EtOAc/petroleum ether, 9:1
[α]D20=+10.5° (c. 0.8, CHCl3
1H NMR (CDCl3) δ (ppm) 1.90 (s, 3H, NCOCH3), 2.29 (s, 3H, SCOCH3), 2.48 (dd, J = 5.7, 12.9Hz, 1H, β Ha cys), 2.82 (dd, J = 6.4, 12.9Hz, 1H, β Hb cys), 2.92–3.01 (m, 2H, NCH2CH2S), 3.32–3.42 (m, 2H, NCH2CH2S), 4.07–4.20 (m, 1H, α H cys), 5.70 (d, J = 7.6Hz, 1H, NH cys), 6.34 (t, J = 5.5Hz, 1H, NHCH2), 7.19–7.35 and 7.40–7.47 (2m, 15H, aromatic H)
MS (FAB +/NBA + K +) m/z 545 (M + K)+, 507 (M + H)+; (FAB-/NRA) m/z 505 (M-H)−
Analysis Calc. for C28H30N2O3S2 (506): C, 66.40; H, 5.93; N, 5.53. Found: C, 66.17; H, 6.00; N, 5.81
2...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright page
- Dedication
- Introduction
- Chapter I: Acquired Immune Deficiency Syndrome
- Chapter II: Addiction Disorders
- Chapter III: Alzheimer's Disease
- Chapter IV: Analgesics
- Chapter V: Antibacterial Agents
- Chapter VI: Anti-inflammatory Agents
- Chapter VII: Autoimmune Disorders
- Chapter VIII: Cardiovascular Disorders
- Chapter IX: Diabetes
- Chapter X: Diagnostics
- Chapter XI: Epilepsy
- Chapter XII: Gastrointestinal Disorders
- Chapter XIII: Hepatitis C
- Chapter XIV: Hormonal Disorders
- Chapter XV: Immunosuppressants
- Chapter XVI: Improved Synthetic Methods
- Chapter XVII: Incontinence
- Chapter XVIII: Irritable Bowel Syndrome
- Chapter XIX: Malaria
- Chapter XX: Migraine Headaches
- Chapter XXI: Obesity
- Chapter XXII: Ocular Disorders
- Chapter XXIII: Osteoporosis
- Chapter XXIV: Parkinson's Disease
- Chapter XXV: Proliferative Disorders
- Chapter XXVI: Psychiatric
- Chapter XXVII: Skin Disorders
- Chapter XXVIII: Sleep Disorders
- Chapter XXIX: Thyroid Disorders
- Chapter XXX: Tinnitus
- Appendix: Patent Assignees
- Index