
- 284 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Muscular Dystrophies
About this book
The Handbook of Clinical Neurology Vol 101: Muscular Dystrophies discusses the pathogenesis and treatment prospects for muscular dystrophies. It summarizes the advances in molecular and cell biology, biochemistry, and other biological sciences, with an emphasis on their application to this group of muscle disorders and to their clinical implications.Starting with an overview of muscular dystrophies, the book's 16 chapters discuss dystrophinopathies; sarcoglycanopathies; congenital muscular dystrophies; collagen VI-related myopathies; limb-girdle muscular dystrophy 2A; dysferlinopathies; limb-girdle muscular dystrophy 2H and the role of TRIM32; and caveolinopathies. The book also covers myofibrillar myopathies; Emery–Dreifuss muscular dystrophy; facioscapulohumeral dystrophy and scapuloperoneal syndromes; oculopharyngeal muscular dystrophy; myotonic dystrophy types 1 and 2; and distal muscular dystrophies.This book is useful to basic investigators, as it offers an increased understanding of muscular dystrophies; and to clinicians, with its emphasis on issues that are relevant to the care, diagnosis, and management of patients with these disorders.- Valuable insights into the muscular dystrophies, including treatment, diagnosis, and care and patient management- A comprehensive compilation of the combined wisdom of the most highly regarded physicians, experts, and scientists studying the muscular dystrophies- An evaluation of the way advances in molecular and cell biology, biochemistry, and other biological sciences continue to advance the study of these disorders
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Introduction
Classification
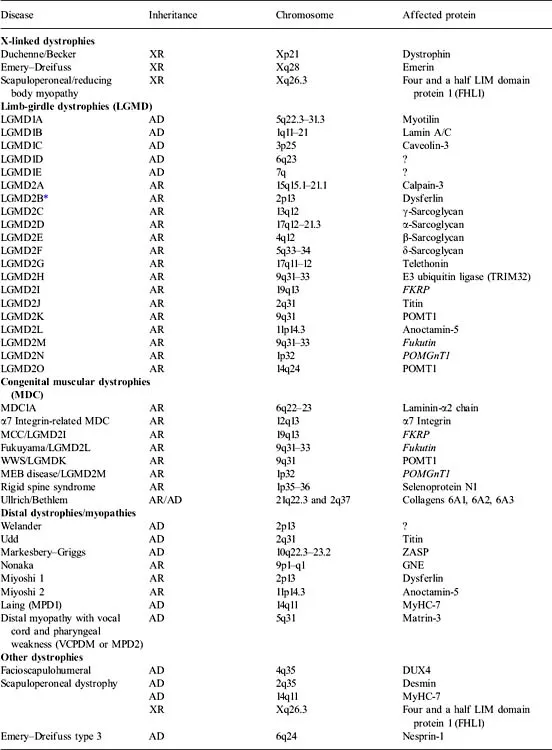
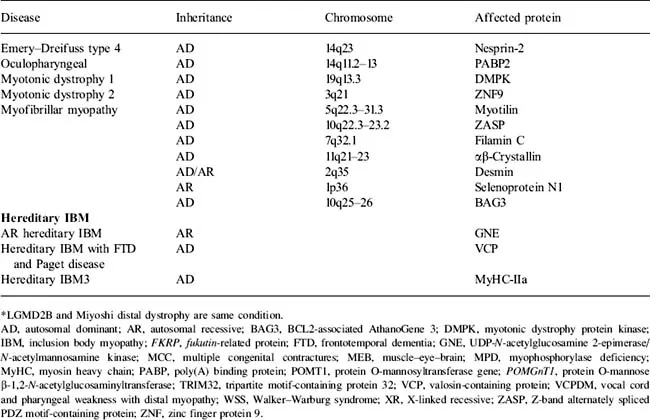
Molecular pathogenesis for dystrophies
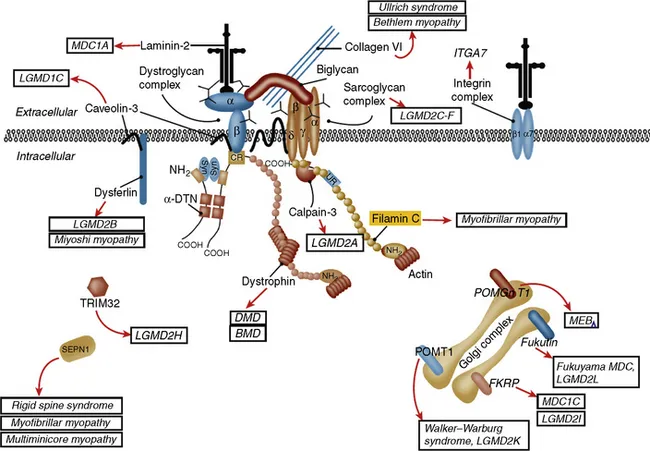
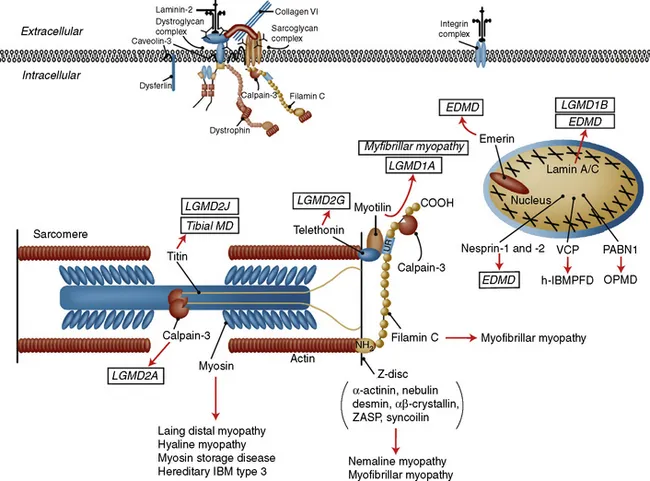
Table of contents
- Cover
- Contents
- Series Editors
- Copyright
- Handbook of Clinical Neurology 3rd Series
- Foreword
- Preface
- List of Contributors
- Chapter 1: Overview of the muscular dystrophies
- Chapter 2: Dystrophinopathies
- Chapter 3: Sarcoglycanopathies
- Chapter 4: Congenital muscular dystrophies
- Chapter 5: The collagen VI-related myopathies
- Chapter 6: Limb-girdle muscular dystrophy 2A
- Chapter 7: Dysferlinopathies
- Chapter 8: Other limb-girdle muscular dystrophies
- Chapter 9: Limb-girdle muscular dystrophy 2H and the role of TRIM32
- Chapter 10: Caveolinopathies
- Chapter 11: Myofibrillar myopathies
- Chapter 12: Emery–Dreifuss muscular dystrophy
- Chapter 13: Facioscapulohumeral dystrophy and scapuloperoneal syndromes
- Chapter 14: Oculopharyngeal muscular dystrophy
- Chapter 15: Myotonic dystrophy types 1 and 2
- Chapter 16: Distal muscular dystrophies
- Index