
- 262 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Biology at the Single Molecule Level
About this book
This is the first book solely devoted to single-molecule biochemistry and molecular biology. Authors were selected on the basis of their contribution to this new and exciting field, and were asked to focus more on the biological problems that can be approached using single-molecule techniques rather than on the techniques per se. It is thought that such techniques will eventually dominate the physical characterization of biologically important macromolecules.
Trusted by 375,005 students
Access to over 1 million titles for a fair monthly price.
Study more efficiently using our study tools.
Information
Part I
reprinted from Progress in Biophysics & Molecular Biology 74/1-2
Protein structural dynamics by single-molecule fluorescence polarization
Joseph N. Forkey*, Margot E. Quinlan and Yale E. Goldman, School of Medicine, University of Pennsylvania, Physiology Department, Pennsylvania Muscle Institute, D700 Richards Building, 3700 Hamilton Walk, Philadelphia, PA 19104-6083, USA *
Corresponding author.
Keywords
Single molecule
Fluorescence
Polarization
Evanescent wave
Microscopy
Bifunctional
Dynamics
Total internal reflection
Contents
1 Introduction
1.1 Protein rotational motions
1.2 Fluorescence polarization
1.3 Single-molecule fluorescence polarization
1.4 Early single-molecule fluorescence experiments
2 Experimental methods
2.1 Fluorescent probes
2.2 Instrumentation
2.2.1 Far-field excitation
2.2.2 Total internal reflection excitation
2.2.3 Near-field optical probe excitation
2.2.4 Microscope objectives and emission optics
2.2.5 Detectors
3 Analysis of single-molecule fluorescence polarization data
3.1 Stationary fluorophores
3.1.1 Absorption polarization ratios
3.1.2 Emission polarization ratios
3.2 Non-stationary molecules
3.2.1 Fast wobble
3.2.2 Slow wobble
3.3 Measurement of the axial angle, θ
4 Applications
5 Conclusions
Acknowledgements
Appendix A Dependence of polarized fluorescence intensity on molecular orientation .
A.1 Excitation of a single fluorophore
A.2 Fluorescence from a single fluorophore
References
1 Introduction
The astonishing progress made during the last two decades toward understanding the machinery of cells has been fueled in large part by developments in structural biology, molecular biology, and biophysics. Thousands of protein structures have been solved at atomic resolution and the amino acid sequences and roles of many specific motifs are now known. While application of these techniques has greatly enhanced our understanding of the structure and biochemistry of proteins, elucidation of the quantitative relationships between structural changes in the proteins, enzymatic activity, signaling, energy conversion and macromolecular interactions will require further development of novel methods.
In this chapter we describe a new technique, single-molecule fluorescence polarization (SMFP), that has the potential to fill a void in conventional methods for determining the structural basis of protein activity. Using state-of-the-art optical microscopes and novel fluorescent probes attached to specific protein domains, SMFP makes it possible to quantify angular rotations that mediate function in individual protein molecules. To demonstrate the importance of protein domain rotational motions, this chapter begins with several examples of biophysical systems in which orientational changes are essential to their mechanism. This is followed by a discussion of the advantages of detecting such rotations with individual molecules and a brief synopsis of the development of SMFP and early experiments. The next two sections are devoted to technical considerations, including characteristics of extrinsic fluorescent probes, optical apparatus and data analysis. Finally, recent work using this technique is reviewed.
1.1 Protein rotational motions
Crystallographic studies of proteins trapped in several different configurations analogous to states of the functioning system often lead to hypotheses for the structural basis of the catalytic activity. They also identify likely sites of interaction with upstream and downstream functional partners. Distinct conformations are typically characterized by differences in the relative orientations of nearby compact domains linked by hinges or swivels composed of glycine residues or flexible loops. The implied rotations have direct bearing on the functional output as illustrated here by several examples where large orientation changes have been discovered.
Due to the helical structure of double-stranded DNA (dsDNA), nucleic acid processing enzymes produce a torque when unwinding the helix to gain access to the base sequence. These enzymes catalyze processes such as replication, transcription, repair and recombination.
Topoisomerase II removes accumulated strain in dsDNA and enables separation of chromosomes or untangling of circular DNA after replication. It operates by passing one dsDNA segment through a ‘gate’ temporarily created in another double-stranded segment. During passage of the intact dsDNA through the gate, quaternary structural changes in the DNA-opening platform take place. Dramatic reorganization of accessory protein domains include rotations of up to 170° (Fig. 1a; Fass et al., 1999).
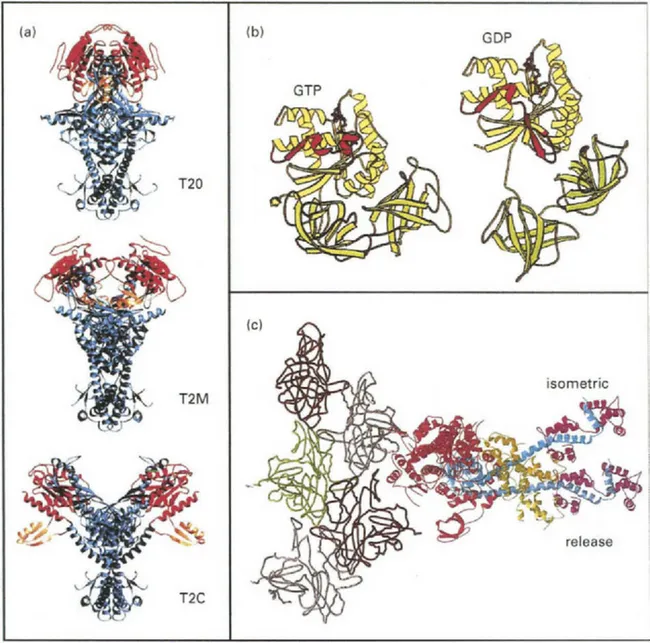
Fig. 1 Examples of crystal structures showing protein domain rotations. (a) Topoisomerase II (T2). Both the Rossman fold (red) and the B’ hook (orange) domains rotate relative to the DNA binding platform (blue) during activity. (b) Ribosomal elongation factor-Tu undergoes a large nucleotide-hydrolysis-dependent reorientation of domains. Note the relative positions of domain I (yellow) versus domains II and III (green) and the transition from α-helix to β-strand in Switch I (red). (c) Crystal structure of chicken skeletal muscle myosin subfragment-1 (red, blue, tan, and magenta) docked to actin (gray, brown and green; Rayment et al., 1993a). The angle of the light chain domain has been modified to show the relative orientation change determined from fluorescence polarization experiments with bifunctional rhodamine (see Section 2.1) when a muscle fiber is suddenly allowed to shorten during isometric contraction. (a) (Reprinted from with permission from Fass et al., 1999, Structural Biology, Nature 6, 322–326.); (b) (Reprinted from FEBS Letters 452, Clark et al., “Structural information for explaining the molecular mechanism of protein biosynthesis” pp. 41–46 © 1999 with permission from Elsevier Science.); (c) (Reprinted from Corrie et al. “Dynamic measurement of myosin light-chain-domain tilt and twist in muscle contraction”, Nature 400, 425–430 © 1999 with permission from Nature (http://www.nature.com/).
During protein biosynthesis, two GTP-binding elongation factors (EF-Tu and EF-G) enable the ribosome to accurately translate the genetic code into amino acid sequences and to maintain the codon reading frame. In each elongation cycle of a nascent polypeptide, an aminoacylated transfer RNA (tRNA) binds to the ribosome as a ternary complex with EF-Tu and GTP. If the tRNA’s anti-codon matches the codon in the template messenger RNA, EF-Tu hydrolyzes the GTP and dissociates. The amino acid is then transferred from the tRNA to the peptide. Crystal structures show a nearly 90° rotation between domains in EF-Tu accompanying GTP hydrolysis (Fig. 1b; Clark et al., 1999). The tRNA also rotates ∼60° relative to the ribosome. EF-G participates in translocation of the mRNA and two tRNA molecules by exactly 3 bases along the synthetic machinery. Whether EF-G functions as a ‘motor’ and whether it exhibits large domain rotations are controversial (Rodnina et...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Editorial: Single-molecule biochemistry coming of age
- Part I: reprinted from Progress in Biophysics & Molecular Biology 74/1-2
- Part II: reprinted from Progress in Biophysics & Molecular Biology 77/1
- This page is intentionally left blank
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Biology at the Single Molecule Level by S.H. Leuba,J. Zlatanova in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biochemistry. We have over one million books available in our catalogue for you to explore.