
eBook - ePub
ABC Proteins
From Bacteria to Man
- 530 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
ABC Proteins
From Bacteria to Man
About this book
ABC Proteins is an in-depth, up-to-date analysis of all that is known about the subject to date. It discusses and compares evolution, biology and mechanism of action of all known ABC proteins, including the first structural studies as well as clinical implications. It will be useful to anyone trying to stay abreast of the latest findings. This book is sure to become a classic and will regularly be updated.- Phylogeny and Evoloution of ABC Transporters- Fundamental Aspects of the Mechanism of Action of ABC Transporters- Prokaryote ABC Transporters- Non-Mammalian Transporters- Multidrug Transporters- ABC Transporters, Physiological Roles and Human Disease- Full color throughout
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
At the moment all of our mobile-responsive ePub books are available to download via the app. Most of our PDFs are also available to download and we're working on making the final remaining ones downloadable now. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access ABC Proteins by I Barry Holland,Susan P. C. Cole,Karl Kuchler,Christopher F. Higgins in PDF and/or ePUB format, as well as other popular books in Médecine & Toxicologie. We have over one million books available in our catalogue for you to explore.
Information
PART I
PHYLOGENY AND EVOLUTION OF ABC TRANSPORTERS
CHAPTER 1
PHYLOGENETIC AND FUNCTIONAL CLASSIFICATION OF ABC (ATP-BINDING CASSETTE) SYSTEMS*
ELIE DASSA
This paper is dedicated to the memory of Maurice Hofnung (1942–2001), a pioneer in the study of ABC (ATP-binding cassette) systems. Two decades ago, by noticing a strong sequence similarity between HisP and MalK, the two first-described ABC proteins, he initiated the studies that led to the identification and characterization of this large superfamily.
INTRODUCTION
ATP-binding cassette (ABC) systems constitute one of the most abundant families of proteins. At the time of writing this review, we have identified more than 2000 ABC ATPase domains or proteins in translated nucleic acid sequence databases. A total of about 6000 proteins were found when the partners of ATPases were taken into account. The size of this mass of sequences is therefore similar to the coding capacity of a bacterial genome. Several properties of members of this superfamily have been reviewed in the last decade (Ames and Lecar, 1992; Ames et al., 1990, 1992; Doige and Ames, 1993; Higgins, 1992; Higgins et al., 1988; Holland and Blight, 1999). The most prominent characteristic of these systems is that they share a highly conserved ATPase domain, the ABC, which has been demonstrated to bind and hydrolyze ATP, thereby providing energy for a large number of biological processes. The amino acid sequence of this cassette displays three major conserved motifs, the Walker A and Walker B motifs commonly found in ATPases together with a specific signature motif, usually commencing LSGG-, and also known as the linker peptide (Schneider and Hunke, 1998). The crystal structures of some ABC proteins are presented in Chapters 4 and 7.
ABC systems are involved not only in the import or export of a wide variety of substances, but also in many cellular processes and in their regulation. Importers constitute mainly the prokaryotic transporters dependent upon a substrate-binding protein (BPD), whose function is to provide bacteria with essential nutrients even if the latter are present in submicromolar concentrations in the environment (Boos and Lucht, 1996). Exporters are found in both prokaryotes and eukaryotes and are involved in the extrusion of noxious substances, the secretion of extracellular toxins and the targeting of membrane components (Fath and Kolter, 1993). The third type of ABC system is apparently not involved in transport but rather in cellular processes such as DNA repair, translation or regulation of gene expression. Since ATP is found principally in the cytosol, we define import as the inwardly directed transport of a molecule into the cytosol. By contrast, export is the translocation of a molecule out of the cytosol, even if its final location is an intracellular organelle. ABC systems of the three types can be distinguished on the basis of the design of their component parts. All the transporters are composed of four structural domains: two very hydrophobic membrane-spanning or integral membrane domains (IMs) and two hydrophilic cytoplasmic domains containing the ABC, peripherally associated with IM on the cytosolic side of the membrane. (a) Importers have in general the four domains encoded as independent polypeptides and they need for function an extracellular substrate-binding protein. (b) In most well-characterized exporters, the transmembrane domains are fused to the ABC domains in several ways. However, some systems with separated IM and ABC domains have been reported to act as exporters although the complete characterization of their transport mechanism awaits more studies. Prokaryote exporters also require accessory proteins and these will be discussed in the specific sections dealing with these transporters. (c) Systems involved in cellular processes other than transport do not have IM domains and are composed of two ABC domains fused together.
INVENTORY AND CLASSIFICATION OF ABC SYSTEMS
To understand the complexity and diversity of ABC systems, computer-assisted methods have been applied by several authors based on comparisons of the ABC ATPase domain, the most highly conserved element. These methods were instrumental in the early definition of the superfamily on the basis of primary sequence comparisons (Higgins et al., 1986). However, in most cases, the ABC proteins of a given organism (Braibant et al., 2000; Linton and Higgins, 1998; Quentin et al., 1999) or ABC systems with clear functional similarity (Fath and Kolter, 1993; Hughes, 1994; Kuan et al., 1995) were compared. The presence of the highly conserved ATPase domain permitted more global comparisons, for example (Paulsen et al., 1998). The first general phylogenetic study specifically devoted to the ABC superfamily (Saurin et al., 1999) was recently updated to include the analysis of about 600 ATPase proteins or domains (Dassa and Bouige, 2001). The sequences segregate in 33 clusters on the phylogenetic tree shown in Figure 1.1. Some clusters comprise obviously highly related proteins known to function together; for example, the two ATPases of oligopeptide importers were fused into a single family. The final 29 families are listed in Table 1.1. Since a general nomenclature for ABC systems is not yet available, Table 1.1 provides the present nomenclature and the equivalent alternative adopted for transporters in general (Saier, 2000) or specifically for human ABC systems (see Chapter 3).
TABLE 1.1
CLASSES, FAMILIES AND SUBFAMILIES OF ABC SYSTEMS
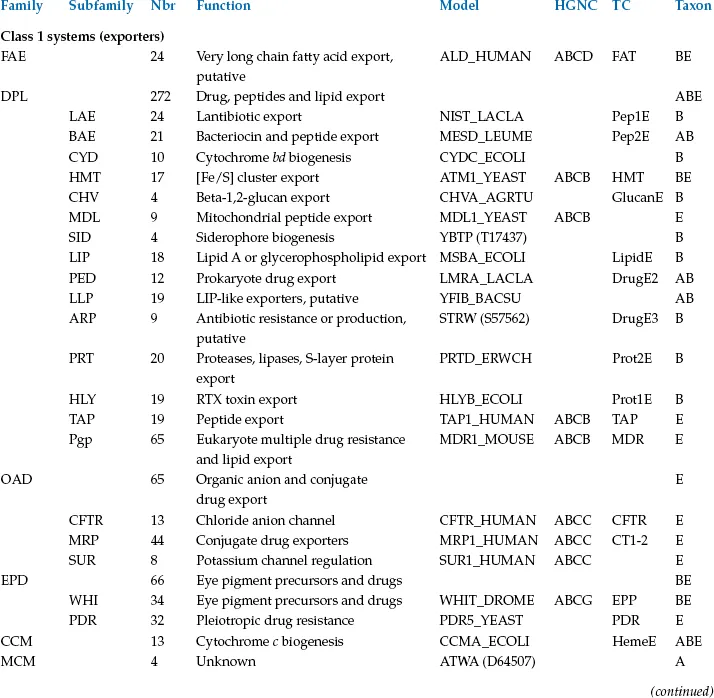
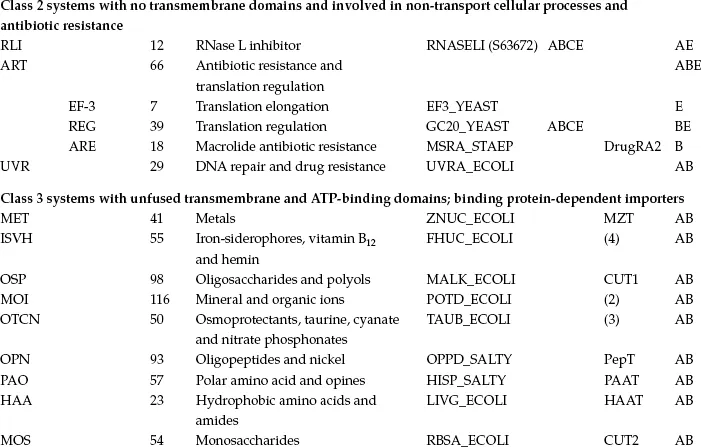
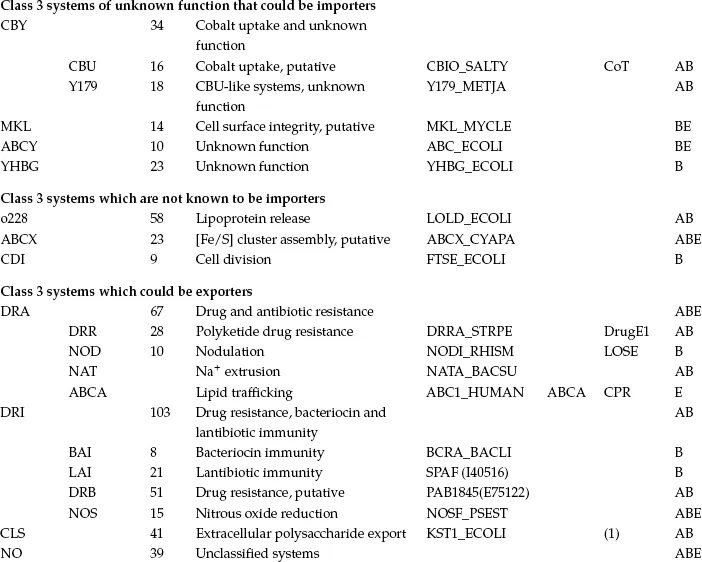
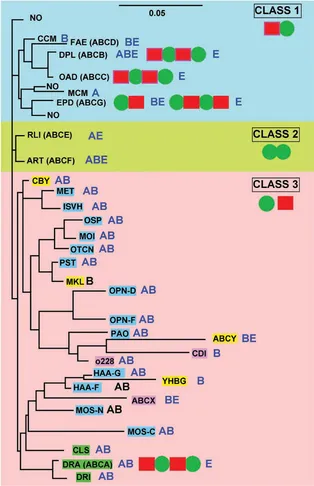
Figure 1.1 Unrooted simplified phylogenetic tree of ABC proteins and domains. For the sake of clarity, only the branches pointing to families have been drawn. The major subdivisions of the tree are indicated according to the nomenclature used in the text. Class 1: systems with fused ABC and IM domains (exporters); class 2: systems with no known transmembrane domains (antibiotic resistance, translation, etc.); class 3: systems with IM and ABC domains carried by independent polypeptide chains (BPD importers and other systems). Under the name of the class, the minimal consensus organization of ABC systems is represented by colored symbols in a linear fashion. IM proteins or domains are represented by red rectangles and ABC proteins or domains by green circles. When the organization of a system in a family does not fit exactly with the consensus, it is indicated on the same line as the system name. In class 3, BPD transporters are highlighted in blue, while systems that are not conclusively related to import are highlighted in purple; systems that could be importers are colored in yellow and systems that could be exporters in green. The sequences of UVR family proteins were omitted from this analysis (see the section on the UVR family for details). Family names are abbreviated according to the conventions used in Table 1.1 and throughout the text and the nomenclature of human ABC systems is given in parentheses after the name of the family. NO represents a few sequences with unknown function and apparently unrelated to neighboring families. They are not discussed in the text. OPN-D, OPN-F; HAA-F, HAA-G and MOS-N, MOS-C correspond to the two different ABC subunits of OPN, HAA and MOS systems, respectively. The distribution of the systems in the three kingdoms of life is indicated as follows: A (archaea), B (bacteria) and E (eukaryotes). The scale at the top of the figure corresponds to 5% divergence per site between sequences.
FAMILIES OF ABC SYSTEMS IN LIVING ORGANISMS
This classification was derived solely on the basis of the comparison of the s...
Table of contents
- Cover
- Title page
- Table of Contents
- Copyright
- CONTRIBUTORS
- THE EDITORS
- ABC TRANSPORTERS: AN INTRODUCTION AND OVERVIEW
- PART I: PHYLOGENY AND EVOLUTION OF ABC TRANSPORTERS
- PART II: FUNDAMENTAL ASPECTS OF THE MECHANISM OF ACTION OF ABC TRANSPORTERS
- PART III: PROKARYOTE ABC TRANSPORTERS
- PART IV: NON-MAMMALIAN EUKARYOTE ABC TRANSPORTERS
- PART V: MAMMALIAN MULTIDRUG RESISTANCE ABC TRANSPORTERS
- PART VI: ABC TRANSPORTERS, PHYSIOLOGICAL ROLES AND HUMAN DISEASE
- Index