![]()
SECTION II
Organ Systems
Chapter 3. Autophagy in the Cardiovascular System
Chapter 4. Autophagy in the Immune System
Chapter 5. Autophagy in the Gastrointestinal Tract
Chapter 6. Autophagy in the Homeostasis of Pancreatic β-Cells
![]()
Chapter 3
Autophagy in the Cardiovascular System
Kazuhiko Nishida, Ph.D.1, Osamu Yamaguchi, M.D. Ph.D.1 and Kinya Otsu, Ph.D.1,2
1Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, Suita, Osaka, Japan
2Correspondence to Kinya Otsu MD. PhD., Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine, 2-2 Yamadaoka, Suita 565-0871, Osaka, Japan
Introduction
Heart disease is the leading cause of death in the industrialized world1. Heart disease of diverse sorts culminates in heart failure, a syndrome wherein the heart is unable to meet the metabolic demands of the body2. Five million Americans currently suffer from chronic heart failure with a mortality of approximately 50% at five years. The heart is an organ of remarkable plasticity, and adult cardiomyocytes are terminally differentiated and replication-deficient2. In response to pathological stress from neurohumoral activation, hypertension or other myocardial injury, the heart initially compensates with an adaptive hypertrophic increase in cardiac mass in order to normalize ventricular wall stress. However, cardiac hypertrophy is an independent risk factor for cardiovascular mortality3 and is a major factor for heart failure4, 5. Under prolonged stress, the heart undergoes apparently irreversible changes culminating in heart failure. Although several theories exist regarding the mechanism governing the transition from hypertrophy to heart failure6, the precise mechanism remains elusive.
Autophagy has evolved as a conserving process for bulk degradation and recycling of cytoplasmic components, such as long-lived proteins and organelles7-9. Autophagy is controlled by autophagy-related genes (Atgs), many of which, including Atg5, Atg7 and Beclin 1, are involved in autophagosome formation. Recent studies have demonstrated a variety of physiological and pathophysiological roles in autophagy such as adaptation to nutrient deprivation, intracellular clearance of proteins and organelles, elimination of micro-organelles including mitochondria, and maintenance of endoplasmic reticulum (ER). Paradoxically, autophagy also appears to modulate cell death through excessive self-digestion and degradation of essential cellular constituents10, 11. In the heart, the level of autophagy is altered in response to stresses triggered by cardiovascular diseases such as cardiac hypertrophy12 and heart failure13, 14. The multiple features of apoptosis, necrosis and autophagy have been simultaneously observed in failing human hearts15, 16. Since it is unclear whether autophagy is protective or detrimental, it is imperative to have a full understanding of the cell death mechanism regarding not only apoptosis and necrosis but also autophagy in cardiomyocytes.
Autophagy in the Heart at a Low Basal Level Under Normal Conditions
Cardiomyocytes are mitochondria-rich, and damaged mitochondria release proapoptotic factors such as cytochrome c17. Autophagy can remove the damaged mitochondria and prevent activation of apoptosis18, 19.
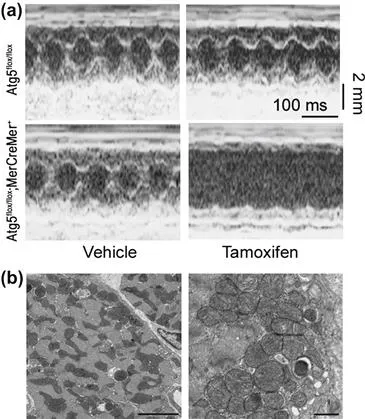
In previous reports regarding autophagy in the heart’s basal state, LAMP2-deficient mice showed excessive accumulation of autophagic vacuoles and impaired autophagic degradation of long-lived proteins, resulting in cardiomyopathy14, 20. In our examination, autophagy also appears to play a protective role under normal or mildly stressed conditions, in the heart’s so-called basal state. Temporally controlled and cardiac-specific Atg5-deficiency in tamoxifen-treated Atg5flox/flox;MerCreMer+ mice21 leads to left ventricular dilatation and contractile dysfunction (Figure 3.1a)22. Ultra-structural analyses of Atg5-deficient hearts reveal a disorganized sarcomere structure, misalignment and aggregation of mitochondria, and aberrant concentric membranous structures (Figure 3.1b). Inactivation of Atg5 causes the accumulation of abnormal proteins and organelles and promotes ER stress and apoptosis22.
FIGURE 3.1 Cardiac dysfunction in tamoxifen-treated Atg5flox/flox;MerCreMer+ mice.(22) a) Echocardiographic analysis. b) Electron micrographs of tamoxifen-treated Atg5flox/flox;MerCreMer+ mouse hearts. Scale cars: 5μm in left, 1μm in right.
Autophagy also plays a role in cardiac aging. Abnormal proteins and damaged mitochondria accumulate during aging23, 24. In cardiomyocytes, the production of reactive oxygen species from mitochondria increases with age and leads to more mitochondrial damage25. In our examination, the cardiac-specific Atg5-deficient mice died earlier than the wild-type mice due to cardiac dysfunction with a disorganized sarcomere structure and collapsed mitochondria26. These results indicate that constitutive cardiomyocyte autophagy is required for protein quality control and normal cellular structure and function under a basal state. Accumulation of abnormal proteins and organelles, especially mitochondria, may directly cause cardiac dysfunction27.
Autophagy in Cardiac Hypertrophy
Cardiac hypertrophy is the consequence of an increase in cardiomyocyte size. Since cardiomyocytes have little or no capacity for cellular proliferation, their only means of growth is by hypertrophy. In response to stress from neurohumoral activation, hypertension or other myocardial injury, however, the heart initially compensates with an adaptive hypertrophic increase in cardiac mass in order to normalize ventricular wall stress. This increase is characterized by enhanced protein synthesis and an increase in the size and organization of cardiomyocyte sarcomeres2, 28. The compensated hypertrophic response is beneficial. However, prolonged stress leads to irreversible decompensation, culminating in dilatation and contractile dysfunction. This is accompanied by thinning of the ventricular walls through a combination of proteolysis and/or cardiomyocyte cell death29.
Autophagy has been observed in hypertrophied myocardium30. Protein turnover is increased during hypertrophy, although in previous reports, autophagy was diminished in response to aortic stenosis12 and isoproterenol infusion31. Pressure overload is accompanied by an elevated rate of myocardial protein synthesis and cardiac hypertrophy32. In our experiments, pressure overload due to transverse aortic constriction (TAC) induced hypertrophy without cardiac dysfunction 1 week after TAC in wild-type mice33. Autophagic activity was suppressed in TAC-induced hypertrophied hearts at the 1-week time point compared with that in sham-operated hearts22. This suggests that autophagic activity is reduced during compensative hypertrophic response27.
We also have reported that temporally controlled and cardiac-specific Atg5-deficiency in tamoxifen-treated Atg5flox/flox;MerCreMer+ mice leads to cardiac hypertrophy22. Knockdown of Atg7 by adenovirus expressing short hairpin RNA targeted to Atg7 inhibits autophagy in rat neonatal cardiomyocytes and then induces cardiomyocyte hypertrophy with typical characteristics22. Rapamycin, a potent activator of autophagy, prevents cardiac hypertrophy induced by thyroid hormone treatment34, or aortic banding35, and regresses existing cardiac hypertrophy36. This suggests that autophagy can also decrease cardiac mass and antagonize cardiac hypertrophy by increasing protein degradation. However, cardiac hypertrophic response is similar between cardiac-specific Atg5-deficient mice and control mice after TAC22. Zhu H et al reported that autophagic activity increases rapidly after severe TAC and is maintained at elevated levels for at least three weeks37, whereas we reported that autophagic activity is suppressed in TAC-induced hypertrophied hearts as mentioned above22. Although mice that are haploinsufficient for Beclin 1 (Beclin 1+/-) have partial suppression of autophagic activity, cardiac hypertrophic response is similar between Beclin 1+/- mice and control mice three weeks after TAC37.
These results suggest that autophagy does not play an important role in regulating cardiomyocyte hypertrophy induced by hemodynamic stress or that its function in the hypertrophic process is compensated by the action of other hypertrophic signaling mechanisms. The role of autophagy in cardiac hypertrophy remains to be elucidated.
Autophagy in Heart Failure
Autophagy has been observed in failing myocardium caused by dilated cardiomyopathy13, 15, 38, by valvular disease39, and by ischemic heart disease40-42. In human failing hearts with idiopathic dilated cardiomyopathy, the prevalence of autophagic, apoptotic and necrotic cells also has been observed15. In animal models, dead and dying cardiomyocytes showing characteristics of autophagy also have been observed. In our experiments, pressure overload due to TAC induced heart failure four weeks after TAC in wild-type mice33. Autophagy was up-regulated in failing wild-type hearts at the four-week time point22, 27. However, the question remains as to whether autophagy is a sign of failed cardiomyocyte repair or is a suicide pathway for the failing cardiomyocytes.
We examined the role of autophagy in cardiac remodeling during sustained pressure overload. Cardiac-specific Atg5 deficiency was associated with cardiac dysfunction and left ventricular dilatation one week after TAC22. Polyubiquitinated proteins accumulated, ER stress was increased and apoptosis was promoted in Atg5-deficient hearts. We also examined the role of autophagy against β-adrenergic stimulation in the heart. Infusion of isoproterenol for seven days in cardiac-specific Atg5 deficient mice led to greater left ventricular dilatation and contractile dysfunction compared to wild-type mice. Furthermore, adult cardiomyocytes isolated from cardiac-specific Atg5 deficient mouse hearts were more susceptible to isoproterenol than were those from control hearts. These results indicate that autophagy in failing hearts is an adaptive response to protect cells either from pressure overload or from isoproterenol stimulation22.
Conversely, the pathological remodeling by severe pressure overload is moderately diminished in Beclin 1+/- mice37. In the mice engineered for forced over-expression of Beclin 1 in cardiomyocytes, pressure overload can trigger an amplified autophagic response and pathological remodeling is more severe. This suggests that autophagy can be a maladaptive response.
These experimental o...