I. Introduction
The responses of forest ecosystems to elevated concentrations of atmospheric CO2 cannot be measured directly in experiments that approximate environmental conditions of the future. Even if large and expensive facilities are constructed for manipulation of the abiotic environment in a forest, the edaphic and biotic adjustments that will occur over decade time frames cannot be reproduced in an experimental system. Nevertheless, there is a compelling need to understand the responses of forests to elevated CO2 because of the prominent role of forests in the global carbon cycle and the potential for CO2 fertilization to alter the relationship between the carbon cycle and the climate system ( Solomon and Cramer, 1993).
Our ability to measure forest response is constrained by two prominent features of forests that do not similarly constrain research on other ecosystems. The first constraint is that the dominant organisms of forests—trees—are large and have a long life span, precluding simple pot experiments for assessing growth responses to elevated CO2, as well as many of the important physiological responses. Such studies have been invaluable with crop plants and grasses, where the plant can be grown to its full size and life span. With trees we must instead rely on extrapolation from relevant physiological and morphological indicators of response, and multiyear studies must be conducted in the field so that conclusions are not confounded by the artifactual restrictions of root growth in pots ( Eamus and Jarvis, 1989).
The second important constraint is that the ecological complexity of forest ecosystems greatly increases the importance of considerations beyond single tree responses. Of course, assessment of the response of any ecosystem must encompass considerations of many biotic and abiotic influences besides the response of a single dominant plant species, but the larger spatial and temporal scales of forest ecosystems increase the importance of these considerations—and the difficulty in addressing them—manyfold. In systems of smaller stature, intact ecosystems can be enclosed in chambers and ecosystem-level responses to CO2 measured directly ( Oechel et al., 1991; Drake, 1992; Owensby et al., 1993), but the scale of forests precludes similar studies. Instead, the CO2 responses of critical components of the forest, including interactions and feedback between trees and multiple environmental resources, must be studied separately in a manner conducive to reassembly in an integrated analysis. Scale is an especially important consideration in a complex and diverse forest type such as the eastern North American deciduous forest, raising the issue of differential responses of competing tree species to changes in the atmospheric CO2 concentration ( Bazzaz and Miao, 1993).
Although these problems limiting our ability to obtain data on tree responses that are relevant to ecosystem and global-scale questions seemed daunting, we began a series of experiments in 1981 designed to investigate the responses of trees, especially deciduous species, to elevated CO2 (Norby et al., 1994). The overriding question was whether nutrient limitation, as commonly occurs in unmanaged forests, would preclude growth enhancement in response to CO2 enrichment. The experiments focused on mechanisms of CO2 X nutrient interactions, especially on belowground processes such as exudation, rhizosphere microbial activity, and symbiotic relationships that could alter nutrient availability. The results showed that below-ground processes are often especially stimulated by CO2 enrichment and that nutrient deficiency does not necessarily preclude growth responses to elevated CO2, at least during experiments lasting several months. The mechanisms sustaining growth response under nutrient-limited conditions can include increased nutrient availability in the plant–soil system, as well as decreased physiological demand for nutrients by the plant ( Norby et al., 1986, 1994). Although questions at a finer scale of resolution (e.g., biochemical mechanisms of response) naturally occurred during the course of these experiments, the most important new questions that developed concerned whether short-term responses could be sustained over several growing seasons under field conditions. These are key issues that control whether the growth chamber studies are at all useful in predicting forest responses. The questions that arose included the following: Does feedback between growth and photosynthesis limit the enhancement of photosynthesis over time? How does growth in elevated CO2 alter the processes of nutrient and carbon storage and retranslocation? Do CO2-induced changes in tissue chemistry influence decomposition and nutrient turnover?
Here we describe the field experiment that was designed to test and extend the concepts developed in the series of growth chamber experiments. We will discuss the results of the field experiment in relation to the larger issue of forest ecosystem response to elevated CO2, and finally we will suggest how our new insights should logically lead to new experimental approaches.
II Experimental Approach
In order to begin to address these questions, it was necessary to grow trees in elevated CO2 for more than one growing season under conditions more closely resembling the forest environment than is possible in a growth chamber experiment with potted plants. Hence, an open-top chamber experiment was initiated in 1989. There were three primary objectives of this field experiment:
1. To determine whether the short-term responses of tree seedlings to elevated CO2 are sustained over several growing seasons under field conditions.
2. To compare the responses to elevated CO2 of Liriodendron tulipifera L. (yellow poplar or tulip tree, family Magnoliaceae) and Quercus alba L. (white oak, family Fagaceae). These species are important, cooccurring components of the deciduous forest of eastern North America that have many contrasting physiological, morphological, and ecological features. For example, yellow poplar grows faster than white oak, initiates new leaves throughout the growing season, is more nutrient demanding, and is less drought resistant, and its litter decomposes more rapidly.
3. To provide data and insights relevant for predicting forest ecosystem responses to elevated CO2. Because of the experimental limitations discussed earlier, simulation models will be necessary to predict forest ecosystem responses. These modeling approaches will be much more useful and believable if they incorporate the best evidence about how forest processes, resource interactions, and biogeochemical feedback will be influenced by elevated CO2.
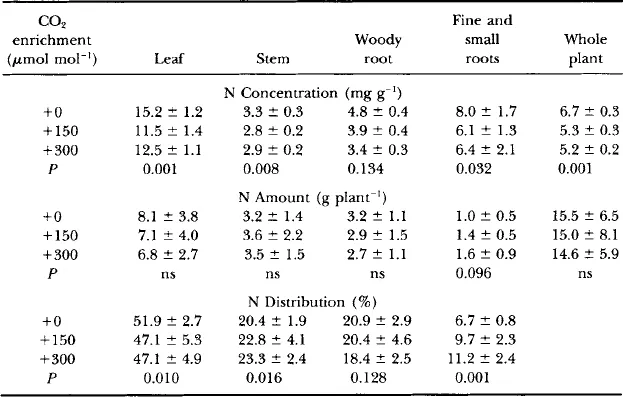
Seeds of the two species were collected from single trees in Fall 1988, germinated, and planted in pots. The seedlings initially were raised in growth chambers containing CO2 concentrations of 380, 500, or 650 μmol mol−1. After a dormant period at 0–4°C, the seedlings were planted in soil within six open-top chambers in May 1989. The chambers were of standard design ( Rogers et al., 1983), 3 m in diameter and 2.4 m tall (later extended to 3.6 m tall). Ten seedlings of each species were planted in each chamber, and later the plots were thinned to five of each species. The faster growing yellow poplar plants were in the northern half of the chamber so as not to shade the white oak plants. Four chambers were provided with CO2 continuously to maintain CO2 concentrations of 150 and 300 μmol mol−1 higher than ambient (two replicate chambers per treatment, designated + 150 and +300). In addition, there were two chambers with no added CO2 (ambient CO2 or +0). Carbon dioxide enrichment was not continued during the winter when the trees were leafless and dormant (mid-November to mid-April). No fertilization or supplemental irrigation was provided during the experiment. Nitrogen mineralization at this site is estimated to be about 100 kg ha−1 yr−1. Foliar N concentrations of the yellow poplar trees ( Table I) were lower than the range considered typical for the species ( Leaf, 1973), suggesting possible N limitation. A period of low rainfall in spring of the second year was reflected in lower stomatal conductance ( Gunderson et al., 1993), but otherwise rainfall was plentiful, there were no morphological manifestations of drought stress, and growth was vigorous.
Table I
Nitrogen Content and Distribution in Yellow Poplar Saplings after Three Growing Seasons in Ambient or Elevated CO2 Concentrations a
aData are the means (±SD) of five plants per chamber in two replicate chambers per CO2 concentration. Nitrogen concentrations of fine (<2 mm) and small (2–7 mm) roots were determined on samples collected from soil cores; these data were converted to grams of N per plant as described in Fig. 2.
Measurements made during the course of the experiment included stem height and basal diameter, from which stem dry mass could be estimated, light-saturated photosynthesis, and stomatal conductance. Leaves were occasionally sampled for constituent analysis. At the end of each growing season, leaves were collected as they abscised. These senescent leaves were measured for determination of final leaf area of the plants and for litter ...