
- 192 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Mixed oxides are the most widely used catalyst materials for industrial catalytic processes. The principal objective of this book is to describe systematically the mixed oxide catalysts, from their fundamentals through their practical applications. After describing concisely general items concerning mixed oxide and mixed oxide catalysts, two important mixed oxide catalyst materials, namely, heteropolyacids and perovskites, are taken as typical examples and discussed in detail.These two materials have several advantages: 1. They are, respectively, typical examples of salts of oxoacids and double oxide, that is, the two main categories of mixed oxides in solid state chemistry. 2. Both exhibit excellent catalytic performance in nearly crystalline state and are used in several industrial applications. 3. They have studied for many years.In addition, metal oxides functioning as a catalyst support (carrier) are included. Although the supports are very important in practical applications, and tremendous progress has been made in the past decades, few systematic reviews exist. It is notable that heteropolyacids and perovskite exhibit unique performance when used as a support.Fundamental catalytic science and technology and solid state chemistry necessary is presented for the proper understanding of mixed oxide catalysts as well as for R&D. For the latter, the concept of design of practical catalysts is very important. This is considered throughout the book.- Systematically describes design principles of mixed oxide catalysts- Shows how catalysis and solid-state chemistry of metal oxides are inter-related- Covers all useful basic concepts of mixed oxide catalysis
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Chapter 1
Basis of Heterogeneous Catalysis
Abstract
Keywords
1.1 Catalyst and Catalysis
1.1.1 Rate and Equilibrium of Chemical Reaction and Role of Catalyst
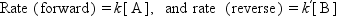
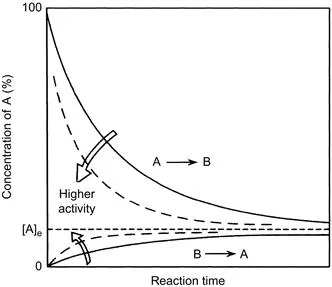
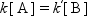
1.1.2 Three Essential Functions of Catalyst
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- Chapter 1. Basis of Heterogeneous Catalysis
- Chapter 2. Chemistry and Catalysis of Mixed Oxides
- Chapter 3. Catalysis of Perovskite and Related Mixed Oxides
- Chapter 4. Catalysis of Heteropoly Compounds (Polyoxometalates)
- Chapter 5. Mixed Oxides as Catalyst Supports
- Index