Pathology and the safety assessment of new medicines
Evaluation of the pathological alterations induced in laboratory animals by novel drugs represents the cornerstone of their safety assessment before they can be first tried in patients. This preliminary assessment, which is based largely on conventional histopathological techniques, represents a major contribution to the development of new treatments for both human and animal diseases.1
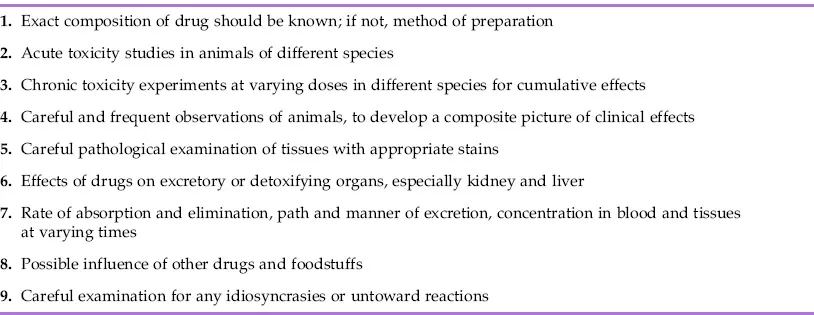
Although there have been many changes in the details of study design and conduct, the principles of drug testing prior to trial in humans are the same as those expounded by Geiling and Cannon after they studied the pathological effects and causes of death of patients treated with a toxic elixir of sulfanilamide over 60 years ago (Table 1.1).2 The basic paradigm of dosing laboratory animals with various doses of a new drug for increasing periods of time accompanied by careful clinical observations, biochemical and hematological monitoring followed by histopathological examination of the tissues remains essentially unaltered and has withstood the test of time. The pathologist is required not only to evaluate alterations to organs and tissues and any relationship that they might have to drug treatment but also to assess likely relevance any treatment-related findings might have for patients.
Table 1.1 Principles of Drug Testing before Trials in Humans as Defined in 1938 by Geiling and Cannon2
The use of animals to study the pathological effects of chemicals and therapeutic agents has a long history. In the 18th century Morgagni reported his attempts to compare pathological changes produced by accidental ingestion of chemicals such as arsenic by people with those induced by administration to animals.3 A thorough and systematic review of pathology induced by toxins in humans and animals was published by Orfila as long ago as 1815.4 Although in the modern era drug safety evaluation has been widely practiced in rodent and non-rodent species since before the Second World War, there have been very few critical comparisons of the effects of drugs in humans and these laboratory animals. Much potentially useful information still resides in archives of pharmaceutical companies and government agencies. Nevertheless, the available data suggest that the traditional approach using experimental pharmacology alongside conventional toxicology studies with pathology is usually sufficient to predict important adverse effects and to support the safe conduct of the first clinical studies in humans.1 Indeed, dosing a rodent and non-rodent species with a new drug up to one month identifies over 90% of adverse effects that will ever be detected in conventional animal studies. However, these animal studies do not predict all adverse drug effects that can occur in clinical practice and there remains significant over- and underprediction of human toxicity. Overall the true positive concordance rate (sensitivity) is of the order of 70% with 30% of human toxicities not predicted by safety pharmacology or conventional toxicity studies.5 Moreover, this concordance varies between different organs and tissues. Therefore each drug-induced pathological finding needs to be assessed on a case-by-case basis for its likely clinical relevance. Moreover, for some systems, histopathology remains crucial, for others it is of lesser importance. For example, animal studies are poor predictors of subjective neurological symptoms but histopathological examination of the nervous system in laboratory animals treated with cancer drugs detects potential serious neurotoxic effects in humans. Likewise pathological examination of the skin in conventional toxicity studies does little to identify important adverse skin hypersensitivity reactions in humans, whereas there appears to be an excellent correlation between the adverse effects in subcutaneous and intramuscular injection sites between animals and humans.1 Animal studies seem to overpredict renal and hepatic toxicity but there is generally a good correlation for gastrointestinal effects. Histopathology still seems to represent one of the most sensitive techniques to detect effects on the reproductive system.6 Nevertheless, the pathologist also needs to be aware that some minor inflammatory alterations in certain organs such as the liver may have greater significance for the use of a drug in humans than particular types of severe damage such as subendocardial necrosis in the myocardium mediated by exaggerated hemodynamic effects.
Treatment-induced findings in conventional toxicity studies found in different laboratory animal species also have variable prognostic value for humans. Although the data are fragmentary, findings in beagle dogs studies appear overall to be better predictors of human adverse effects than data from rodents or surprisingly from primates.1 Dog gastrointestinal and cardiovascular physiology appears to model particularly well for humans.7,8
Another long-standing problem highlighted by the cyclooxygenase 2 (COX-2) inhibitors is the adverse interaction of some therapies with specific human diseases. COX-2 inhibitors were used for inflammatory disorders because of perceived lower side effect profile on the gastrointestinal tract compared with conventional drugs. This benefit was outweighed by an increased incidence of cardiovascular disease in some patients, although withdrawal of such drugs from the market may have reduced the availability of effective treatments for some arthritis patients.9 Similar concerns about an increase in ischemic cardiovascular events with rosiglitazone compared with other non-thiazolidinedione antidiabetic agents has also been the basis for limiting the use of this effective drug by the drug regulatory authorities.10 Such effects are difficult if not impossible to predict from the usual clinical trials let alone conventional toxicity studies. Unfortunately the detection of an increased incidence of a common event such as heart attack or stroke is difficult in patients for it requires a high index of suspicion even though it may have a big impact on public health.11,12 Such interactions usually require randomized controlled trials specifically designed to look for such risks.11 It has to be remembered that aspirin was in use for over 100 years before it became generally acknowledged about 30 years ago to be associated with Reye’s syndrome, a devastating hepatic toxicity in children.13 Although the precise mechanism involved in Reye’s syndrome is unknown it is often preceded by a viral syndrome, usually varicella, gastroenteritis, or an upper respiratory or tract infection such as influenza and it shows a strong epidemiologic association with the ingestion of aspirin.
Veterinary medicines
Similar principles apply to the development and the safety assessment of new medicines for animals, although assessment of environmental impact and residue studies are also required for consumer safety for food-producing animal medicines. While assessment of the relevance of drug-induced pathological findings in laboratory animals requires extrapolation to a wider range of other species, the task is often aided by the ability to conduct toxicity studies at multiples of the therapeutic dose in the target species – but again supported by histopathological examination.14
Toxicological screening
Screening compounds to select the least toxic in a series of chemicals has a long pedigree. In 1909 Paul Ehrlich, looking for a cure for infectious disease, screened a large number of arsenic-containing compounds in mice, guinea pigs and rabbits.15 He discovered that one compound #606 not only killed the syphilis microbe but also cured rabbits with syphilis without causing death. This chemical was marketed as the first effective remedy for syphilis under the name of Salvarsan. Gerhard Zbinden and colleagues made a convincing case for flexible, targeted toxicity studies of a series of related chemicals using standard reference agents and small numbers of animals for short periods of time in the selection of the least toxic candidate new drugs.16 These studies are quite widely practiced but they require careful design, critical selection of models and careful evaluation of pathology. In this respect, pathological evaluation of important organs such as liver and kidney in pharmacology studies conducted in disease models can also provide insights to potential toxicity issues.
Carcinogenesis assessment
The evaluation of the carcinogenic potential of drugs designed for long-term use is often seen as where the pathologist ‘comes into his or her own’. Carcinogenicity studies require the careful diagnosis of diverse tumors and preneoplastic lesions that can occur in rodents. However, the contribution of these studies to human safety is not clear cut. About half of the drugs that have been developed over the past two decades have shown tumorigenicity in rodents.17 If a few genotoxic drugs are excluded, the majority appear to have induced tumors as a consequence of exaggerated or unwanted pharmacodynamic effects at high doses which have not precluded their use in patients for treatment of disease.
Various modes of action have been linked to these tumor types although the underlying mechanisms are often unclear.18–22 However, from a pathological perspective, non-relevant tumors tend to occur at high doses where there is histological evidence of persistent cellular toxicity, exaggerated pharmacodynamic effects or other perturbations of homeostasis.23 By contrast, the evidence of tumorigenic response from dosing a range of potent DNA reactive (genotoxic) carcinogens to rodents suggests that there is clear histological evidence of an increase in malignancy in induced tumors compared with tumors that develop spontaneously. Evidence of a malignant phenotype is the presence of metastases distant from the primary tumor site rather than cytological appearances alone. Moreover there is usually a much earlier age of onset compared with tumors that develop spontaneously and those that follow administration of non-DNA reactive chemicals. A review of the National Toxicology Program (NTP) database also suggested that potent genotoxic carcinogens produce tumors in characteristic multiple sites in rodent studies.24 Much relevant information is scattered in the pathology literature, although there have been a number of pathology reviews of rodent tumor types of questionable significance to humans.18,25–27
In view of these difficulties as well as the resources needed and time involved in conducting a traditional two-year carcinogenicity study in both rats and mice, other approaches have been proposed. It has long been argued that the traditional mouse carcinogenicity study adds little or nothing to the evaluation of carcinogenicity and is consequently a redundant test.28 Monro suggested that, because of improved understanding of rodent tumorigenesis, a single study of 12 to 18 months’ duration in rats alone would be sufficient to identify potential human carcinogens.29 More recent comparisons of results from chronic toxicity studies with carcinogenicity studies performed on a large number of pharmaceutical agents has also suggested that six-month and 12-month toxicity studies are reasonable predictors of tumorigenic outcome in two-year studies.30,31 Cohen has even suggested that critical evaluation of cellular findings in animal studies of merely 13 weeks’ duration can identify many of the chemicals that go on to produce tumors in long-term studies.32 In fact, the prudent pathologist has always evaluated pathology findings in chronic toxicity studies ...