
eBook - ePub
An Introduction to Aspects of Thermodynamics and Kinetics Relevant to Materials Science
- 480 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
An Introduction to Aspects of Thermodynamics and Kinetics Relevant to Materials Science
About this book
This book is based on a set of notes developed over many years for an introductory course taught to seniors and entering graduate students in materials science. An Introduction to Aspects of Thermodynamics and Kinetics Relevant to Materials Science is about the application of thermodynamics and kinetics to solve problems within Materials Science. Emphasis is to provide a physical understanding of the phenomenon under discussion, with the mathematics presented as a guide.
The problems are used to provide practice in quantitative application of principles, and also to give examples of applications of the general subject matter to problems having current interest and to emphasize the important physical concepts.
End of chapter problems are included, as are references, and bibliography to reinforce the text. This book provides students with the theory and mathematics to understand the important physical understanding of phenomena.
- Based on a set of notes developed over many years for an introductory course taught to seniors and entering graduate students in materials science
- Provides students with the theory and mathematics to understand the important physical understanding of phenomena
- Includes end of chapter problems, references, and bibliography to reinforce the text
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access An Introduction to Aspects of Thermodynamics and Kinetics Relevant to Materials Science by Eugene Machlin in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER I
Thermodynamics of Phases having Constant Composition
Publisher Summary
This chapter illustrates thermodynamics of invariant composition phases, such as elements, molecules, line intermetallic compounds, monodisperse colloidal assemblies of hard spheres, and Langmuir monolayers. The chapter emphasizes on relative stability of allotropes and polymorphs of these materials. The set of constraints that apply to the Gibbs free energy and are applicable to most of the phenomena are also examined. Materials exist in either solid, liquid or vapor states. When in the vapor state, a monatomic gas consists of atoms that do not interact and that move freely throughout the container that defines the volume of the gas. Thus, in this state the gas has no potential energy. Its energy is in the form of kinetic energy of the atoms. The potential energy is the sum over the interaction energies between atoms or molecules situated at the minima in their potential wells. The kinetic energy is the sum over the motional energy in vibrations, rotations, and translation of the atoms or molecules. Electrons also contribute to both forms of energy since as temperature increases; there are empty quantum states available for the outer electrons to occupy with a net increase in energy of each atom. These aspects of the states of materials affect their thermodynamic properties in that the configurational and thermal contributions differ in type in the different states and materials.
Introduction
Application of thermodynamics and kinetics to phenomena of interest in materials science is the theme of this book. Most materials in the phrase “materials science” consist of phases or mixtures of phases. By “phase” is meant the homogeneous configuration of atoms or molecules corresponding to a liquid; or to some crystalline solid, such as a solid having the body-centered-cubic (bcc) structure; or to an amorphous solid, such as glass; or to a vapor. A phase has a set of properties including thermodynamic potentials, such as entropy, enthalpy and Gibbs free energy. The phase may or may not be in an equilibrium state. In the equilibrium state the thermodynamic properties of a given phase are not functions of its past history, but are unique. A state of equilibrium is defined for a closed system by the condition that the entropy is at a maximum. Related to this condition it is also defined by the existence of a minimum in one of the thermodynamic potentials listed in Table 1.1 along with the corresponding variables that are constrained to be constant in the minimization procedure.
Table 1.1
Thermodynamic potentials corresponding to stable equilibrium.
| Thermodynamic potential | Constrained variables |
| Gibbs free energy (G) | P,T,N |
| Helmholtz free energy (F) | V,T,N |
| Enthalpy (H) | P,S,N |
| Internal energy (E) | V,S,N |
| Grand (Ω) | V,T,μ |
At equilibrium, there are a variety of variational properties of the thermodynamic potentials. For example, the bulk modulus is given by –V[∂2G/∂V2]T. A listing of the relations between derivatives of thermodynamic parameters and physical properties of materials is given in Table 1.2. The symbols in the table are defined in the list of symbols at the end of this chapter. From examination of this table it is apparent that a knowledge of the dependence of a thermodynamic potential on pressure or temperature of a material will provide a basis for determining a number of material properties. However, there is a more important reason for wanting this knowledge which relates to the relative stability of competing allotropes or polymorphs of a given material.
Table 1.2
Summary of thermodynamic relations.a
| X | Y | Z | 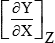 | X | Y | Z | 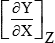 |
| T | V | p | αV | T | p | V | α/β |
| T | S | p | Cp/T | T | S | V | Cp/T – α2V/β |
| T | V | p | Cp – αpV | T | U | V | Cp – α2VT/β |
| T | H | p | CP | T | H | V | Cp – α2VT/β + αV/β |
| T | F | p | − αpV – S | T | F | V | −S |
| T | G | p | −S | T | G | V | αV/β – S |
| p | V | T | −βV | T | p | S | Cp/αVT |
| p | S | T | −αV | T | V | S | −βCp/αT + αV |
| p | U | T | βpV – αVT | T | U | S | βpCp/αT – αpV |
| p | H | T | V – αVT | T | H | S | Cp/αT |
| p | F | T | β... |
Table of contents
- Cover image
- Title page
- Table of Contents
- Preface to Third Edition
- CHAPTER I: Thermodynamics of Phases having Constant Composition
- CHAPTER II: Thermodynamics of Solid Solutions
- CHAPTER III: Free Energy and Phase Diagrams
- CHAPTER IV: Thermodynamics of Interfaces
- CHAPTER V: Heterophase and Homophase Fluctuations
- CHAPTER VI: Thermodynamics of Defects
- CHAPTER VII: Concepts in Kinetics in Solids
- CHAPTER VIII: Diffusion
- CHAPTER IX: Nucleation and Growth Kinetics
- CHAPTER X: Solid–Solid Interface Migration Kinetics
- CHAPTER XI: Growth of Phases: Diffusion or Interface Reaction Control
- CHAPTER XII: Morphological Instability and Growth of Phases
- CHAPTER XIII: Thermodynamics, Kinetics and Patterns
- CHAPTER XIV: Thermodynamics of Micelles
- Index