
- 452 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Polypropylene: The Definitive User's Guide and Databook presents in a single volume a panoramic and up-to-the-minute user's guide for today's most important thermoplastic. The book examines every aspectùscience, technology, engineering, properties, design, processing, applicationsùof the continuing development and use of polypropylene. The unique treatment means that specialists can not only find what they want but for the first time can relate to and understand the needs and requirements of others in the product development chain. The entire work is underpinned by very extensive collections of property data that allow the reader to put the information to real industrial and commercial use. Despite the preeminence and unrivaled versatility of polypropylene as a thermoplastic material to manufacture, relatively few books have been devoted to its study. Polypropylene: The Definitive User's Guide and Databook not only fills the gap but breaks new ground in doing so.Polypropylene is the most popular thermoplastic in use today, and still one of the fastest growing. Polypropylene: The Definitive User's Guide and Databook is the complete workbook and reference resource for all those who work with the material. Its comprehensive scope uniquely caters to polymer scientists, plastics engineers, processing technologists, product designers, machinery and mold makers, product managers, end users, researchers and students alike.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1 Chemistry
1.1 Polymerization reaction
Polypropylene is prepared by polymerizing propylene, a gaseous byproduct of petroleum refining, in the presence of a catalyst under carefully controlled heat and pressure. [773] Propylene is an unsaturated hydrocarbon, containing only carbon and hydrogen atoms:
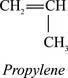
In the polymerization reaction, many propylene molecules (monomers) are joined together to form one large molecule of polypropylene. Propylene is reacted with an organometallic, transition metal catalyst (see 1.4 Catalysts for a description of catalysts used in the reaction) to provide a site for the reaction to occur, and propylene molecules are added sequentially through a reaction between the metallic functional group on the growing polymer chain and the unsaturated bond of the propylene monomer:
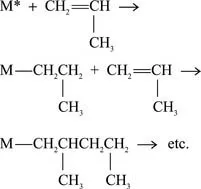
One of the double-bonded carbon atoms of the incoming propylene molecule inserts itself into the bond between the metal catalyst (M in the above reaction) and the last carbon atom of the polypropylene chain. A long, linear polymer chain of carbon atoms is formed, with methyl (CH3) groups attached to every other carbon atom of the chain (Figure 1.1). Thousands of propylene molecules can be added sequentially until the chain reaction is terminated. [764, 768]
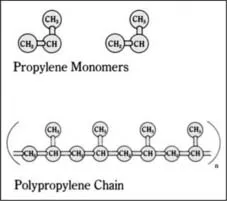
Figure 1.1 Molecules of propylene and polypropylene. In the polymerization reaction, propylene monomers (top) are added sequentially to the growing polymer chain (bottom), to form a long, linear polymer chain composed of thousands of propylene monomers. The portion of the chain shown in parentheses is repeated n number of times to form the polymer. [642]
1.2 Stereospecificity
With Ziegler-Natta or metallocene catalysts, the polymerization reaction is highly stereospecific. Propylene molecules add to the polymer chain only in a particular orientation, depending on the chemical and crystal structure of the catalyst, and a regular, repeating three-dimensional structure is produced in the polymer chain [763]. Propylene molecules are added to the main polymer chain, increasing the chain length, and not to one of the methyl groups attached to alternating carbon atoms (the pendant methyl groups), which would result in branching. Propylene molecules are usually added head-to-tail and not tail-to-tail or head-to-head. Head-to-tail addition results in a polypropylene chain with pendant methyl groups attached to alternating carbons; in tail-to-tail or head-to head addition, this alternating arrangement is disrupted. [771]
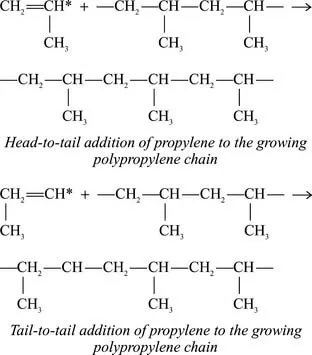
Occasional tail-to-tail or head-to-tail additions of polypropylene to the growing polymer chain disrupt the crystalline structure and lower the melting point of the polymer; formulations in which this occurs are used in thermoforming or blow molding. [694]
Polypropylene can be isotactic, syndiotactic, or atactic, depending on the orientation of the pendant methyl groups attached to alternate carbon atoms. In isotactic polypropylene (Figure 1.2), the most common commercial form, pendant methyl groups are all in the same configuration and are on the same side of the polymer chain. Due to this regular, repeating arrangement, isotactic polypropylene has a high degree of crystallinity. In syndiotactic polypropylene, alternate pendant methyl groups are on opposite sides of the polymer backbone, with exactly opposite configurations relative to the polymer chain. Syndiotactic polypropylene is now being produced commercially using metallocene catalysts. In atactic polypropylene, pendant methyl groups have a random orientation with respect to the polymer backbone. Amounts of isotactic, atactic, and syndiotactic segments in a formulation are determined by the catalyst used and the polymerization conditions. Most polymers are predominantly isotactic, with small amounts of atactic polymer. New metallocene catalysts make possible other stereochemical configurations, such as hemi-isotactic polypropylene. In this configuration, most pendant methyl groups are on the same side of the polypropylene chain, as in isotactic polypropylene; however, other methyl groups are inserted at regular intervals on the opposite side of the chain. [794, 695, 810]
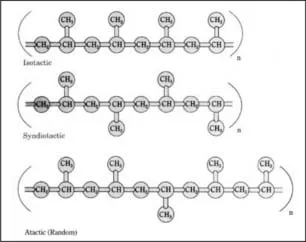
Figure 1.2 Stereochemical configurations of polypropylene. In isotactic polypropylene, top, the pendant methyl groups branching off from the polymer backbone are all on the same side of the polymer backbone, with identical configurations relative to the main chain. In syndiotactic polypropylene, middle, consecutive pendant methyl groups are on opposite sides of the polymer backbone chain. In atactic polypropylene, bottom, pendant methyl groups are oriented randomly with respect to the polymer backbone. The portion of the chain shown is repeated n number of times to form the polymer. [642]
1.3 Effect on characteristics of polypropylene
The structure and stereochemistry of polypropylene affect its properties.
1.3.1 Stereochemistry
Because of its structure, isotactic polypropylene has the highest crystallinity, resulting in good mechanical properties such as stiffness and tensile strength. Syndiotactic polypropylene is less stiff than isotactic but has better impact strength and clarity. Due to its irregular structure, the atactic form has low crystallinity, resulting in a sticky, amorphous material used mainly for adhesives and roofing tars. [794, 691] Increasing the amount of atactic polypropylene in a predominantly isotactic formulation increases the room temperature impact resistance and stretchability but decreases the stiffness, haze, and color quality. [695] The amount of atactic polypropylene in a polypropylene formulation is indicated by the level of room temperature xylene solubles; levels range from about 1–20%. [771] Polypropylenes generally have...
Table of contents
- Cover Image
- Title page
- Copyright
- Figures
- Graphs
- Tables
- Foreword and Acknowledgements
- Table of Contents
- Introduction
- Chapter 1: Chemistry
- Chapter 2: Morphology and Commercial Forms
- Chapter 3: Additives
- Chapter 4: Fillers and reinforcements
- Chapter 5: Films
- Chapter 6: Sheets
- Chapter 7: Fibers
- Chapter 8: Foams
- Chapter 9: Recycling
- Chapter 10: Safety and Health
- Chapter 11: Applications
- Chapter 12: Design principles
- Chapter 13: Processing fundamentals
- Chapter 14: Injection molding
- Chapter 15: Blow molding
- Chapter 16: Extrusion
- Chapter 17: Thermoforming
- Chapter 18: Fabricating and Finishing
- Chapter 19: Polypropylene Data Collection
- Glossary of Terms
- Index
- Sources
- Polypropylene Supplier Directory
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Polypropylene by Clive Maier,Theresa Calafut in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.