eBook - ePub
Sol-Gel Science
The Physics and Chemistry of Sol-Gel Processing
- 912 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Sol-Gel Science: The Physics and Chemistry of Sol-Gel Processing presents the physical and chemical principles of the sol-gel process. The book emphasizes the science behind sol-gel processing with a chapter devoted to applications. The first chapter introduces basic terminology, provides a brief historical sketch, and identifies some excellent texts for background reading. Chapters 2 and 3 discuss the mechanisms of hydrolysis and condensation for nonsilicate and silicate systems. Chapter 4 deals with stabilization and gelation of sols. Chapter 5 reviews theories of gelation and examines the predicted and observed changes in the properties of a sol in the vicinity of the gel point. Chapter 6 describes the changes in structure and properties that occur during aging of a gel in its pore liquor (or some other liquid). The discussion of drying is divided into two parts, with the theory concentrated in Chapter 7 and the phenomenology in Chapter 8. The structure of dried gels is explored in Chapter 9. Chapter 10 shows the possibility of using the gel as a substrate for chemical reactions or of modifying the bulk composition of the resulting ceramic by performing a surface reaction (such as nitridation) on the gel. Chapter 11 reviews the theory and practice of sintering, describing the mechanisms that govern densification of amorphous and crystalline materials, and showing the advantages of avoiding crystallization before sintering is complete. The properties of gel-derived and conventional ceramics are discussed in Chapter 12. The preparation of films is such an important aspect of sol-gel technology that the fundamentals of film formation are treated at length in Chapter 13. Films and other applications are briefly reviewed in Chapter 14. Materials scientists and researchers in the field of sol-gel processing will find the book invaluable.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Sol-Gel Science by C. Jeffrey Brinker,George W. Scherer in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Physical & Theoretical Chemistry. We have over one million books available in our catalogue for you to explore.
Information
CHAPTER 1
Introduction
Our goal is to present the fundamental principles of sol-gel processing. This requires that we explore areas of physics (e.g., fractal geometry and percolation theory), chemistry (mechanisms of hydrolysis and condensation), and ceramics (sintering and structural relaxation) that may be unfamiliar to many readers, including those involved in sol-gel research. Therefore we introduce each topic on an elementary level and develop it only so far as necessary to make our point; numerous references are provided for those who want more detail. In this chapter we begin by stepping through the stages of the process, which parallel the structure of the book, and by introducing the terminology. Then we provide a brief historical sketch of the evolution of this field of study plus a list of “essential reading.”
Figure 1 presents a schematic of the routes that one could follow within the scope of sol-gel processing, with each stage labelled by the number of the relevant chapter in this book. This illustration recurs as a “locator” at the beginning of each chapter to provide a perspective on the place of that step in the overall process.
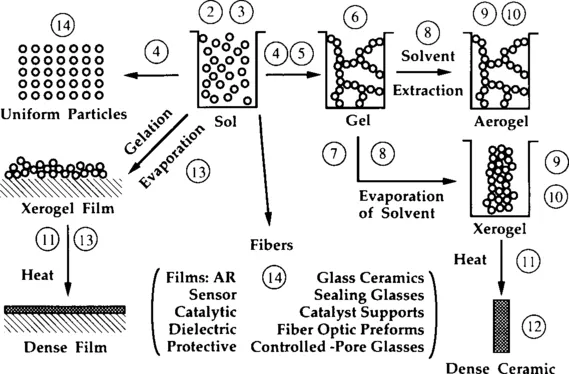
1 SOL-GEL PROCESSING
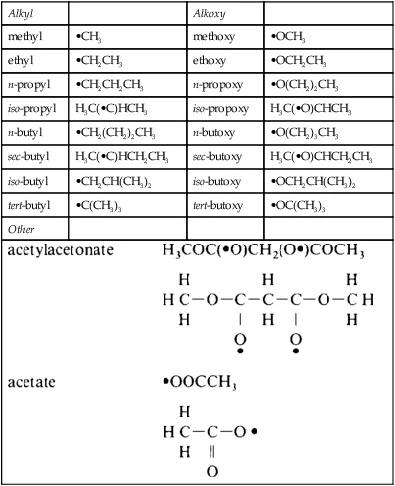
A colloid is a suspension in which the dispersed phase is so small (∼ 1–1000 nm) that gravitational forces are negligible and interactions are dominated by short-range forces, such as van der Waals attraction and surface charges. The inertia of the dispersed phase is small enough that it exhibits Brownian motion (or Brownian diffusion), a random walk driven by momentum imparted by collisions with molecules of the suspending medium. A sol is a colloidal suspension of solid particles in a liquid. An aerosol is a colloidal suspension of particles in a gas (the suspension may be called a fog if the particles are liquid and a smoke if they are solid) and an emulsion is a suspension of liquid droplets in another liquid. All of these types of colloids can be used to generate polymers or particles from which ceramic materials can be made. A ceramic is usually defined by saying what it is not: it is nonmetallic and inorganic; some would also say it is not a chalcogenide. We thus include all metal oxides, nitrides, and carbides, both crystalline and noncrystalline. In the sol-gel process, the precursors (starting compounds) for preparation of a colloid consist of a metal or metalloid element surrounded by various ligands (appendages not including another metal or metalloid atom). For example, common precursors for aluminum oxide include inorganic (containing no carbon) salts such as Al(NO3)3 and organic compounds such as Al(OC4H9)3. The latter is an example of an alkoxide, the class of precursors most widely used in sol-gel research. An alkane is a molecule containing only carbon and hydrogen linked exclusively by single bonds, as in methane (CH4) and ethane (C2H6); the general formula is CnH2n+2. An alkyl is a ligand formed by removing one hydrogen (proton) from an alkane molecule producing, for example, methyl (•CH3) or ethyl (•C2H5) (where the dot • indicates an electron that is available to form a bond). An alcohol is a molecule formed by adding a hydroxyl (OH) group to an alkyl (or other) molecule, as in methanol (CH3OH) or ethanol (C2H5OH). An alkoxy is a ligand formed by removing a proton from the hydroxyl on an alcohol, as in methoxy (•OCH3) or ethoxy (•OC2H5). A list of the most commonly used alkoxy ligands is presented in Table 1.
Table 1
Commonly Used Ligands.
| Alkyl | Alkoxy | ||
| methyl | •CH3 | methoxy | •OCH3 |
| ethyl | •CH2CH3 | ethoxy | •OCH2CH3 |
| n-propyl | •CH2CH2CH3 | n-propoxy | •O(CH2)2CH3 |
| iso-propyl | H3C(•C)HCH3 | iso-propoxy | H3C(•O)CHCH3 |
| n-butyl | •CH2(CH2)2CH3 | n-butoxy | •O(CH2)3CH3 |
| sec-butyl | H3C(•C)HCH2CH3 | sec-butoxy | H3C(•O)CHCH2CH3 |
| iso-butyl | •CH2CH(CH3)2 | iso-butoxy | •OCH2CH(CH3)2 |
| tert-butyl | •C(CH3)3 | tert-butoxy | •OC(CH3)3 |
| Other | |||
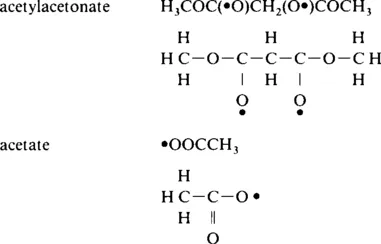 | |||
Dot (•) indicates bonding site. Parentheses indicate atom with available bond. n = normal (meaning a linear chain), sec = secondary, tert = tertiary.
Metal alkoxides are members of the family of metalorganic compounds, which have an organic ligand attached to a metal or metalloid atom. The most thoroughly studied example is silicon tetraethoxide (or tetraethoxysilane, or tetraethyl orthosilicate, TEOS), Si(OC2H5)4. Organometallic compounds are defined as having direct metal-carbon bonds, not metal-oxygen-carbon linkages as in metal alkoxides; thus, alkoxides are not organometallic compounds, although that usage turns up frequently in the literature. Metal alkoxides are popular precursors because they react...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Preface
- Acknowledgments
- Chapter 1: Introduction
- Chapter 2: Hydrolysis and Condensation I: Nonsilicates
- Chapter 3: Hydrolysis and Condensation II: Silicates
- Chapter 4: Particulate Sols and Gels
- Chapter 5: Gelation
- Chapter 6: Aging of Gels
- Chapter 7: Theory of Deformation and Flow in Gels
- Chapter 8: Drying
- Chapter 9: Structural Evolution during Consolidation
- Chapter 10: Surface Chemistry and Chemical Modification
- Chapter 11: Sintering
- Chapter 12: Comparison of Gel-derived and Conventional Ceramics
- Chapter 13: Film Formation
- Chapter 14: Applications
- INDEX