
Structural Biology in Immunology
Structure/Function of Novel Molecules of Immunologic Importance
- 186 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Structural Biology in Immunology
Structure/Function of Novel Molecules of Immunologic Importance
About this book
Structural Biology in Immunology, Structure/Function of Novel Molecules of Immunologic Importance delivers important information on the structure and functional relationships in novel molecules of immunologic interest. Due to an increasingly sophisticated understanding of the immune system, the approach to the treatment of many immune-mediated diseases, including multiple sclerosis, systemic lupus erythematosus, rheumatoid arthritis, and inflammatory bowel disease has been dramatically altered. Furthermore, there is an increasing awareness of the critical role of the immune system in cancer biology. The improved central structure function relationships presented in this book will further enhance our ability to understand what defects in normal individuals can lead to disease.- Describes novel/recently discovered immunomodulatory proteins, including antibodies and co-stimulatory or co-inhibitory molecules- Emphasizes new biologic and small molecule drug design through the exploration of structure-function relationship- Features a collaborative editorial effort, involving clinical immunologists and structural biologists- Provides useful and practical insights on developing the necessary links between basic science and clinical therapy in immunology- Gives interested parties a bridge to learn about computer modeling and structure based design principles
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Organization of Immunological Synapses and Kinapses
† Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom,
‡ New York University School of Medicine, New York, NY, United States
Abstract
Keywords
Acknowledgments
1.1 Introduction
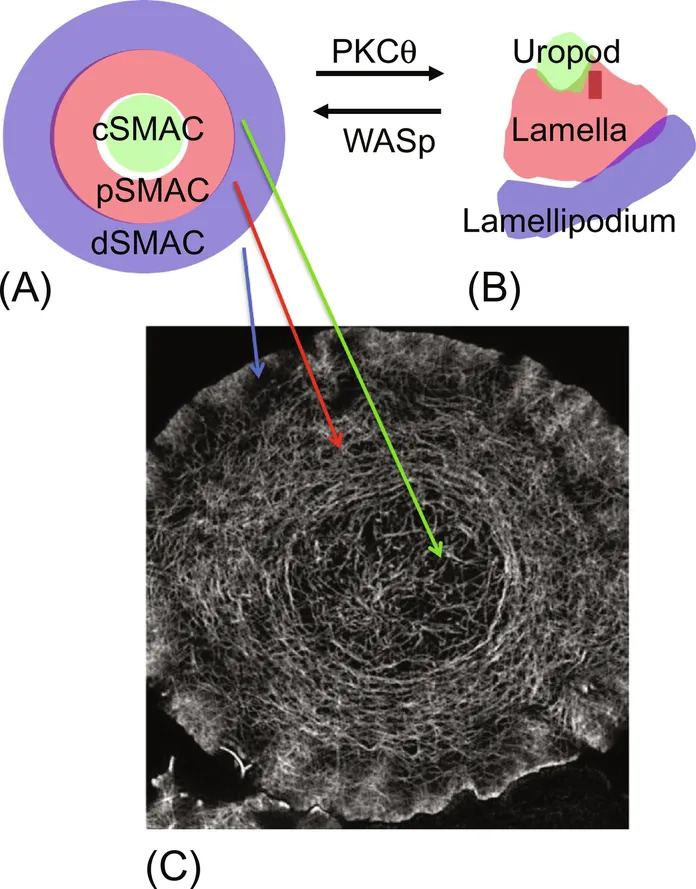
1.2 Receptors and Ligands of the Immunological Synapse and Kinapse
1.2.1 T-cell Receptor and pAgMHC
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Dedication
- Contributors
- Acknowledgments
- Chapter 1: Organization of Immunological Synapses and Kinapses
- Chapter 2: Principles of Protein Recognition by Small T-Cell Adhesion Proteins and Costimulatory Receptors
- Chapter 3: Synthetic Antibody Engineering: Concepts and Applications
- Chapter 4: Natural Killer Cell Receptors
- Chapter 5: Structure-Function in Antibodies to Double-Stranded DNA
- Chapter 6: The Role of the Constant Region in Antibody-Antigen Interactions: Redefining the Modular Model of Immunoglobulin Structure
- Index