
- 269 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
This book provides comprehensive up-to-date information on the structure and function of immunoglobulins. It describes the basic features of these molecules, which assists the reader in understanding how they function as an integral part of the immune system. The Immunoglobulins describes the localization and structure of different binding sites of immunoglobulin molecules, including the antigen-binding site, on the basis of latest x-ray crystallography studies. It discusses recently developed biotechnological methods that allow scientists to obtain fully active antibody molecules in vitro even without immunization and to construct new variants of immunoglobulins and their fragments by fusing with various other active molecules. A survey of recent knowledge on immunoglobulin-binding molecules other than antigens and on flexibility of immunoglobulin molecules concludes the discussion of functional aspects of the problem.
- Describes recent reviews on the structure and function of immunoglobulin molecules of various species
- Summarizes in detail recent findings on the fine structure of the antigen-combining site
- Presents comparative data on the antigen-recognizing sites of other molecules such as MHC proteins and T-cell receptors
- Summarizes growing data on immunoglobulin binding sites responsible for the reaction of immunoglobulins with molecules other than antigens
- Explores the rapid advance of recent biotechnological methods used for the construction of antibody molecules and their fragments with new properties
- Presents extensive references and is lavishly illustrated
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
PART I
Structural Aspects
CHAPTER 1
General Characteristics of Immunoglobulin Molecules
Immunoglobulin molecules of all classes are heterodimers and composed from four polypeptide chains—two identical large or heavy (H) chains of about 50–60 kDa and two small or light (L) chains of about 23 kDa, linked by interchain disulfide bridges (Fig. 1). This prototype structure, first suggested by Rodney Porter in 1962, is common for all monomeric immunoglobulin molecules. Polymeric immunoglobulins of higher molecular weights are formed by 2–6 four-chain subunits, similar to the monomeric immunoglobulin molecules. They possess one or two additional peptide chains, which are important for formation and stabilization of immunoglobulin polymers. All immunoglobulin molecules are glycoproteins and contain two or more carbohydrate chains, usually linked to heavy chains. Membrane forms of immunoglobulins serve as a specific part of the B-cell antigen receptors. They have additional peptide stretches on the COOH-terminal ends of their heavy chains, with which they are embedded in cell membranes, as well as short portions of chains inside the cell (intracellular segments of different sizes). Membrane immunoglobulins are associated noncovalently with two accessory peptides forming the B-cell antigen receptor complex in the B lymphocyte membrane.
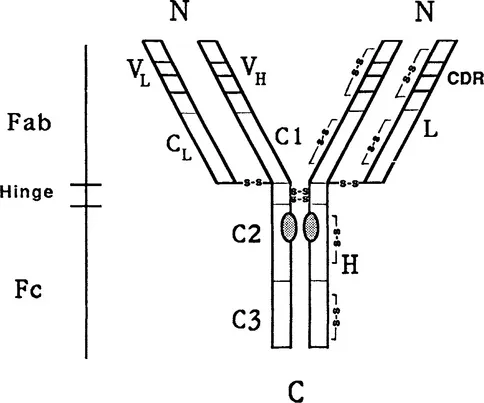
FIGURE 1 The basic four-chain structure of immunoglobulin molecules. H and L, the heavy and light peptide chains; N and C, NH2- and COOH-terminal ends of the peptide chains; VL and VH, variable regions of the light and heavy chains; C1, C2, and C3, homology regions (domains) of the heavy chains; CDR, complementarity-determining region. The distribution of interchain and intrachain disulfide bonds are shown. Shadowed ovals point to localization of oligosaccharides in CH2 domains. (Nezlin, 1994. Reprinted with permission from Marcel Dekker, NY.)
I. IMMUNOGLOBULIN CLASSES
In humans and rodents, there are five immunoglobulin classes or isotypes, which differ in the primary structure, carbohydrate content, and antigenic properties of their heavy chains (Table 1). By contrast, the light chain types are the same for all immunoglobulin classes. Each immunoglobulin molecule contains light chains of one of two types, either lambda (λ) or kappa (κ). The λ and κ light chains have different primary structures and antigenic properties. They are usually free of carbohydrate components. The ratio of κ/λ chains in human and swine immunoglobulins is about 60:40, whereas in mouse, rat and rabbit immunoglobulins it is about 95:5. Some other mammals such as dog, cat, and farm animals (ox, sheep, and horse) have mainly λ chains and chicken immunoglobulins contain only λ chains.
TABLE 1
Properties of Major Immunoglobulin Classes
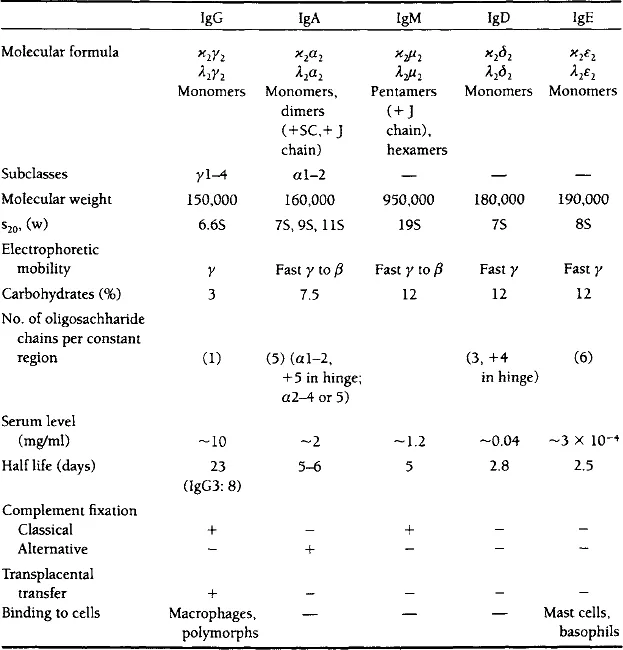
(Nezlin, 1994. Reprinted with permission from Marcel Dekker, NY.)
A. IMMUNOGLOBULIN G
Immunoglobulin G (IgG) is the major class of immunoglobulins. About three-quarters of all serum immunoglobulins belong to this class. IgG molecules consist of two heavy γ and two light chains (2γ + 2L). Normally each molecule of IgG has two identical antigen combining sites. Upon electrophoresis at alkaline pH, IgG migrates slower than almost all other serum proteins. Each IgG molecule contains about 3% carbohydrates, whereas that of other immunoglobulins is usually much higher (8–12%). After a secondary immunization, B lymphocytes secrete predominantly IgG molecules. IgG, unlike other immunoglobulins, can cross the placenta barrier and can penetrate into extravascular areas. IgG molecules are able to react with Fcγ receptors present on the surface of macrophages and some other cells. The interaction with Fc receptors initiates various effector reactions and particularly facilitates the destruction of potentially harmful germs recognized by IgG antibodies. IgG molecules can also activate complement by the classical pathway. In humans and mice there are four IgG subclasses, which differ in the structure of their heavy chains (Table 2) (Shakib, 1990). Other animals have from one (rabbit) and two (sheep) to four (cattle) and five (horse and swine) IgG subclasses.
TABLE 2
Properties of Subclasses of Human Immunoglobulin G
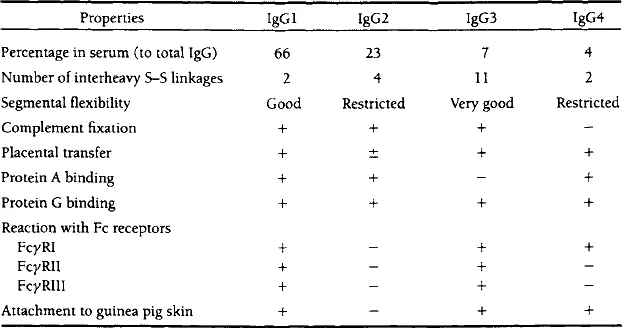
(Nezlin, 1994. Reprinted with permission from Marcel Dekker, NY.)
A cysteine residue near the middle of the human and mouse γ 1 chains (position 220) is involved in the formation of the heavy–light disulfide bridge with the penultimate cysteine residue of the light chains. In other IgG subclasses and other immunoglobulin classes, cysteine residues located between the VH and CH1 domains of the heavy chains (position 131) are involved in the formation of the heavy–light disulfide bridges with the penultimate cysteine residue of light chains. According to the x-ray crystallographic studies, both residues 220 and 131 are in close proximity in the three-dimensional globule, and the heavy chains with both variants of the heavy–light disulfide linkage have the same general structure.
B. IMMUNOGLOBULIN M
Immunoglobulin M (IgM) is a high molecular weight protein (macroglobulin), consisting of five or rarely of six subunits (IgM monomers). Like IgG molecules, the IgM monomers are composed of two heavy and two light chains, which are linked together by disulfide bridges. The IgM monomers are found at a low concentration in human serum. Each pentameric IgM molecule is composed of 10 heavy (μ) chains, 10 light chains and usually one joining (J) chain (Metzger, 1970). The B cells can also secrete functionally active IgM hexamers lacking J chains but the amount of hexamers in serum is no more than 5% of total IgM (Brewer et al., 1994). The carbohydrate content of IgM is high, about 12%. A pentameric IgM molecule has 10-antigen combining sites and can bind 10 small antigens (haptens). However, due to steric restrictions, only five large antigen molecules can be bound by one IgM molecule. The antibody activity of IgM is destroyed upon reduction of the intersubunit disulfide linkages. Such a reduction can easily be achieved with very low concentrations of reducing agents, such as dithiothreitol or mercaptoethanol.
IgM appeared early in evolution and primitive vertebrates have macro-globulins whose structure resembles that of mammalian IgM molecules. IgM are the first immunoglobulins synthesized by neonates and are the preponderant class of immunoglobulin molecules appearing during early phases of immune responses. In the monomeric form, IgM functions as an antigen-specific part of the B-cell antigen receptor on the surface of unstimulated B lymphocytes. The antigen receptors with the participation of the μ chains are very important for the normal development of B cells.
The polymeric IgM molecules are very efficient activators of the classical complement cascade. A single IgM molecule can activate complement component C1, whereas for that several IgG molecules are needed. The hexamer IgM molecules are much more efficient in activation of complement than pentameric IgM but they have nearly the same avidity to antigen as pentamers (Davis et al., 1988; Randall et al., 1990). Due to the multiplicity of their combining sites, IgM are very efficient in agglutination and cytolytic reactions. The so-called natural antibodies are mainly IgM and provide the first protection against invading microorganisms. Some “natural” IgM antibody molecules exhibit antiself activity (Potter and Smith-Gill, 1990; Avrameas, 1991; Mouthon et al., 1996).
The B lymphocytes do not secrete pentameric IgM molecules but express IgM monomers on their surface. The plasma cells secrete only IgM polymers. The mechanism of the retention of monomeric IgM inside cells is linked at least partly with the carboxyl terminus of the ...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- Preface
- PART I: Structural Aspects
- PART II: Functional Aspects
- INDEX
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access The Immunoglobulins by Roald Nezlin in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biochemistry. We have over one million books available in our catalogue for you to explore.