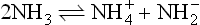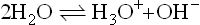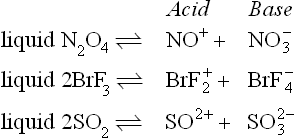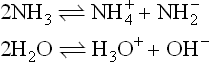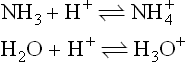![]()
CHAPTER I
THE NATURE AND SCOPE OF INORGANIC NON-AQUEOUS SOLVENTS
Publisher Summary
This chapter focuses on the nature and scope of suitable nonaqueous solvents for inorganic chemistry. There are two considerations that influence the choice of suitable solvent systems: (1) the possibility of dissociating the solute into ions and (2) the possibility of setting up acid–base systems in which the solvent might participate. The application of the acid–base concept has been the dominant theme in the development of nonaqueous solvents in inorganic chemistry. The protonic solvents water and ammonia behave as bases by uniting with a proton. In terms of electron-pair bonding, this requires donation of an electron pair from base to proton. In the Lux–Flood acid–base concept, oxide ions are regarded as the transferable species corresponding to protons in the Lowry–Bronsted scheme. Experimentally, the studies in a nonaqueous solvent require the preparation and purification of the solvent, the means of manipulation, and the methods for the observation of phenomena in it. The means of manipulation depend upon physical properties such as the melting point to boiling point temperature range.
Water is such a common and therefore readily obtainable substance that it was an obvious choice as a solvent by the very early chemists. The extraordinary versatility of water as a solvent was soon recognized and the solubilities of many substances were determined over the range 0–100°C. It was not surprising that other solvents were almost completely neglected until the development of organic chemistry produced, simultaneously, organic substances which were often insoluble in water and organic liquids which could be used as solvents instead of water.
The process by which a simple organic molecule, e.g. a paraffin hydrocarbon, dissolves in (say) benzene is comparatively simple. The relatively weak intermolecular forces between the solute hydrocarbon molecules permit dissolution and are replaced by solvent-solute interactions which, again, are weak; the “driving force” leading to solution is here the change to a state of higher entropy which the solute molecules attain by solution and hence the solubility usually increases markedly with temperature.
By contrast, the process of solution in water is always complicated, and even now it is not in general possible to make quantitative predictions about solubilities. The complications arise because in water there are already relatively strong, specific and directed intermolecular forces — hydrogen bonds — which give to the liquid water some semblance of an ordered crystalline structure. The mechanism by which, for example, an ionic solid
such as sodium chloride dissolves, is not then simply a matter of the reduction of the strong inter-ionic attractions in the crystals by a continuous medium of high dielectric constant. The reorientation of the solvent consequent upon solvation of both cation and anion plays an important role in the energetics of solution. However, covalent solids can sometimes dissolve in water and here hydrogen bonding between solute and solvent is generally the factor favouring solubility; solutes containing —OH or —NH2 groups, such as alcohols, carbohydrates, amines and amino-compounds are typical examples of this type of behaviour.
Although many non-ionic substances undergo hydrolysis in water by an essentially bimolecular process in which a water molecule “attacks” the solute molecule, apparent hydrolysis — appearing as a decrease in pH — is not uncommon in solutions of salts of multivalent ions. Here, however, the change in pH is due to an increased dissociation of water molecules co-ordinated around highly charged cations, i.e. the process is essentially unimolecular.
The frequent occurrence of hydrolysis, real or apparent, does limit the usefulness of water as a solvent; the other limitation is of course the rather narrow liquid range which does not permit the study in solution of substances of low thermal stability or of species stable only at high temperatures. The use of organic solvents to overcome either of these limitations is itself subject to the severe limitation imposed by the very low solubility of many inorganic substances in such solvents; for this reason, organic liquids have not been widely investigated as solvents for inorganic systems, although solvents such as dimethylformamide, dimethyl sulphoxide and the “glymes” (e.g. “diglyme”, 1, 2-dimethoxyethane) are now used to an increasing extent.
The approach to the problem of finding suitable non-aqueous solvents for inorganic chemistry has not in general been made systematically because of the difficulties, already mentioned, which attend any quantitative theoretical approach to the problem of solubility. In practice, two considerations have often influenced the choice of suitable solvent systems; the possibility of dissociating
the solute into ions and the possibility of setting up acid–base systems in which the solvent might participate. Hence solvents possessing a dielectric constant which is greater than about 10 and an electrical conductance, possibly due to some degree of self-ionization, have often been chosen for study. Even a low degree of self-ionization does not, however, preclude the use of a solvent for ionic substances, thus, for example, dinitrogen tetroxide, N2O4, whilst having a low degree of self-ionization can still be used as an effective solvent for many salts.
It is the application of the acid–base concept which has been the dominant theme in the development of non-aqueous solvents in inorganic chemistry. Franklin, who did much pioneering work with liquid ammonia, postulated the following self-ionization equilibrium for this solvent:
compare
He observed acidic behaviour by the ammonium salts (for example NH4Cl) and the basic behaviour of alkali amides (such as potassium amide KNH2) in liquid ammonia and was led to formulate new acid and base definitions; viz. an acid was a substance giving a cation characteristic of the solvent and a base was a substance giving an anion characteristic of the solvent. Otherwise, acids and bases had their characteristic properties, for example, an acid plus a base gave a salt and solvent; acids dissolved metals to produce salts, and so on.
It is important to note that Franklin’s definitions are not restricted to hydrogen-containing substances; thus acid–base behaviour arising from the following possible self-ionization equilibria are covered by them:
Whilst there is good evidence for the first two equilibria, the third is very doubtful, but the important point is that investigations in all three liquids as non-aqueous solvents have been influenced and often aided by Franklin’s definitions as have other investigations of hydrogen-containing solvents, for example liquid hydrogen fluoride (Chapter IV). The weakness of the Franklin definitions is that they are so wide as to be incapable of quantitative application — we cannot use them to compare one solvent with another in any quantitative sense.
If we consider those hydrogen-containing solvents which are protonic,then the familiar Lowry–Brønsted acid–base theory can be applied. Consider again the equilibria
Here the Lowry-Brønsted definition concerns the processes
and the posit...