
- 751 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Fundamentals of Interface and Colloid Science (FICS) is a standard reference work with an educational nature. The emphasis is on the basic facts and phenomena, which are systematically explained. FICS aims to make interface and colloid science accessible to a wide audience. Interface and colloid science is an important and fascinating field, but one that is often overlooked and undervalued. It has applications as diverse as agriculture, mineral dressing, oil recovery, industrial chemistry, medical science and biotechnology.A deductive approach is followed, with systems of growing complexity being treated as the book progresses. Volume I: Fundamentals (1st ed. 1991, 2nd ed. 1993) reviews the physical chemistry required to understand current literature on interfacial and colloid science. The volume starts from first principles and gradually increases the level. Volume II: Solid-Liquid Interfaces (1995) treats the subject systematically for the first time, including adsorption, double layers and electronkinetics. Volume III: Interface Tension covers interfacial tensions, monolayers and wetting.
- Accessible to a wide audience without a detailed knowledge of physics and chemistry
- Complex mathematical derivations are kept to a minimum
- Treats interfacial and colloidal phenomena from first principles (advanced command of physics and chemistry not required)
- Takes the reader from elementary to expert level
- Acts as a reference and a textbook
- Contains extensive and detailed cumulative subject index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Fundamentals of Interface and Colloid Science by J. Lyklema in PDF and/or ePUB format, as well as other popular books in Technology & Engineering & Chemical & Biochemical Engineering. We have over one million books available in our catalogue for you to explore.
Information
1
Interfacial Tension: Measurement
This Volume deals with various aspects of surface tensions and interfacial tensions. Together with the phenomenon of adsorption (enrichment of molecules at interfaces), these tensions constitute the basic characteristics of interfaces.
The concept of surface tension is a very old one. Reportedly, Leonardo da Vinci had already observed and recorded the spontaneous rise of liquids in narrow, wetted capillaries, bores and plugs1). From this rising the phenomenon acquired its name: capillus (lat.) = hair: the bores should be as narrow as a hair. Nowadays the term capillary phenomena is used more widely (in this book also) to indicate not only capillary rise, involving curved interfaces, but also all phenomena determined by the tendency of interfaces to adopt a minimum area, such as drop shapes, bubble shapes, liquid bridges and wetting.
An important historic development was the insight that surface tension is a surface- and not a bulk-property. Newton2) had already discriminated between cohesive and adhesive forces. A decisive discriminative experiment was carried out by Hawksbee3). He investigated liquid rise in capillaries and between glass plates and found that the thickness of the glass did not matter. So the phenomenon of wetting was established as a surface phenomenon, although the depth of the interactions responsible for the various tensions still remains an issue today (chapter 2).
In chapter I.1 we introduced the notions of surface and interfacial tensions phenomenologically.
Volume II mainly dealt with interfaces of solids. For such systems, the adsorption of molecules and ions is the primary phenomenon. Interfacial tensions cannot generally be measured, although interfacial pressures are obtainable from adsorption isotherms using Gibbs’ law.
As a logical sequel, in this third Volume of FICS, liquid-fluid (LG or LL) interfaces will be treated. For such systems the interfacial tension γ is the primary, and measurable, variable. Often the areas involved are so small that the analytical determination of adsorbed amounts (Γ’is) is difficult. In those cases Gibbs’ law can be used to relate interfacial tension to the adsorption, provided adsorbate and solution are in equilibrium with each other. For systems with large LG or LL areas, like foams and emulsions, adsorbed amounts are often more easily measured, but then it must be realized that the surface excesses are to a large extent determined by the history of the samples, and therefore not necessarily representative of the adsorption equilibrium in systems with low areas. Foams and emulsions will be dealt with in Volume V. In line with the systematics of FICS, it is logical to start with the measurement of interfacial tensions. This will be done in the present chapter, emphasizing pure liquids. A selection of results will be tabulated in Appendix 1. This chapter is followed by one on interpretations, two on fluid-fluid interfaces carrying adsorbates, and finally the Volume will be rounded off with a discussion of three-phase contacts, i.e. with wetting phenomena. In view of the scope of FICS, results under ambient conditions of temperature and pressure will be emphasized.
Regarding the terms interface and surface, we follow the convention adopted in Volume I. ‘Interface’ is the general term; the word ‘surface’ is restricted to phase boundaries in which one of the phases is gaseous, or to indicate specifically the outmost part of a condensed phase under consideration (‘the surface of an oil droplet in an oil-in-water emulsion’). In the literature this distinction is not always so systematically maintained. In other places the symbol for interfacial or surface tension is σ or γ. We prefer the latter because σ is widely used for surface charge density, and the two do occur simultaneously in some equations.
Much background information about the notion of interfacial tension, its thermodynamical and statistical interpretation and a number of other aspects have already been dealt with in Volumes I and II1). We start by briefly reviewing these.
1.1 General introduction to capillarity and the measurement of interfacial tensions
For the measurement of interfacial tensions no molecular models are needed. It is enough to know that the interfacial tension is a measure of the tendency of all areas to become as small as possible. Following chapter 1.2, this contractile action can be interpreted thermodynamically or mechanically.
Thermodynamically, the interfacial tension is interpreted as the increase in the Helmholtz or Gibbs energy of the system when the area of the interface under consideration is increased reversibly by an infinitesimal amount dA at constant temperature and composition, and at constant volume or constant pressure, respectively. We can express this as
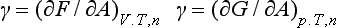
where n is an abbreviation for the set of amounts n1, n2, …., that define the composition of the system. The dimensions of γ are [energy/area]; we shall express them in mJ m−2. As F and G are in principle measurable, so is γ.
Mechanicall...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright page
- General Preface
- Preface to Volume III: Liquid-Fluid Interfaces
- List of Frequently Used Symbols (Volumes I, II and III)
- Chapter 1: Interfacial Tension: Measurement
- Chapter 2: Interfacial Tension: Molecular Interpretation
- Chapter 3: Langmuir Monolayers
- Chapter 4: Gibbs Monolayers
- Chapter 5: Wetting
- Appendices
- Cumulative Subject Index of Volumes I (Fundamentals), II (Solid-Fluid Interfaces) and III (Liquid-Interfaces)