
eBook - ePub
Biophysical Characterization of Proteins in Developing Biopharmaceuticals
- 426 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Biophysical Characterization of Proteins in Developing Biopharmaceuticals
About this book
Biophysical Characterization of Proteins in Developing Biopharmaceuticals is concerned with the analysis and characterization of the higher-order structure (HOS) or conformation of protein based drugs. Starting from the very basics of protein structure this book takes the reader on a journey on how to best achieve this goal using the key relevant and practical methods commonly employed in the biopharmaceutical industry today as well as up and coming promising methods that are now gaining increasing attention.
As a general resource guide this book has been written with the intent to help today's industrial scientists working in the biopharmaceutical industry or the scientists of tomorrow who are planning a career in this industry on how to successfully implement these biophysical methodologies. In so doing a keen focus is placed on understanding the capability of these methodologies in terms of what information they can deliver. Aspects of how to best acquire this biophysical information on these very complex drug molecules, while avoiding potential pitfalls, in order to make concise, well informed productive decisions about their development are key points that are also covered.
- Presents the reader with a clear understanding of the real world issues and challenges in using these methods.
- Highlights the capabilities and limitations of each method.
- Discusses how to best analyze the data generated from these methods.
- Points out what one needs to look for to avoid making faulty conclusions and mistakes.
- In total it provides a check list or road map that empowers the industrial scientists as to what they need to be concerned with in order to effectively do their part in successfully developing these new drugs in an efficient and cost effective manner.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Biophysical Characterization of Proteins in Developing Biopharmaceuticals by Damian J. Houde,Steven A. Berkowitz in PDF and/or ePUB format, as well as other popular books in Biological Sciences & Biochemistry. We have over one million books available in our catalogue for you to explore.
Information
II
The Selected Biophysical Tools in the Biopharmaceutical Industry
Chapter 4
An Introduction and Hierarchical Organization of the Biophysical Tool in Section II
Damian J. Houde, and Steven A. Berkowitz Department of Protein Pharmaceutical Development, Biogen Idec, Inc., Cambridge, MA, USA
Abstract
This chapter provides a brief introduction and hierarchical overview of the biophysical tools to be discussed in Section II of this book. In choosing these tools, we have organized them into a hierarchy of four separate levels (i.e., four tiers). The key criteria for this organization, concerns the richness in information each tool supplies regarding the biophysical properties and higher order structure of a protein drug. In using this key criterion a number of factors were observed to trend with this organization, including: (1) ease to operate and use, (2) cost to implement, (3) required amount of time to upset and acquire data, and (4) level of expertise to process, analyze and interpret data. The biophysical tools at the lowest two tiers (i.e., tier 1 and 2) fall into a general grouping that constitutes the standard or mainstream package of commonly used biophysical tools employed by nearly all biopharmaceutical companies in developing protein drugs. While the biophysical tools in the two highest tiers (i.e., tier 3 and 4), comprise a second grouping of methods that correspond to the more advanced biophysical tools.
Keywords
Advance biophysical tools; Standard biophysical tools4.1. Introduction
In Section I (Chapters 1–3) of this book, details concerning the structure of a protein drug and its various structural elements that contribute to a protein's higher order structure (HOS) were discussed (see Chapter 1). Next, the biophysical characterization of a protein's HOS and how their biophysical properties play an important role in its development into a drug was examined (see Chapter 2). This was then coupled with a brief and general look into the basic biophysical toolbox used to achieve this characterization (see Chapter 3). In proceeding into Section II, the stage is now set to look more carefully and critically into a number of different biophysical tools. In choosing the particular biophysical tools discussed in this section, we have organized and categorized them into four different tiers based on the corresponding increase in informational content that they can provide. Hence, biophysical tools in tier 1, relatively speaking, provide the lowest level of information while biophysical tools in tier 4 provide, again relatively speaking, the highest level of information. In carrying out this categorization, we begin to see other trends as one makes the transition from tier 1 to tier 4 tools. In general, methods derived from tier 1 tools tend to be much easier to use and operate, are less expensive and less costly to implement, require the least amount of time to acquire the data, and need the least training or expertise to process, analyze, and interpret data. However, in moving toward tier 4 tools, the attributes of the methods in each subsequent tier become more difficult to use and operate, are more expensive and more costly to implement, requires more time to acquire the data and more training or expertise to process, analyze, and interpret data.
In creating these four tiers we have further arranged them to form two major groupings as shown in Figure 4.1. In the first grouping, only tier 1 and 2 tools have been included. Biophysical tools in this grouping consist of what we call the “standard” biophysical tools. These tools generally correspond to the most applicable and dominant biophysical tools used by many biopharmaceutical companies in characterizing and developing biopharmaceuticals. Methods generated from these tools form the backbone of many biophysical studies carried out in the biopharmaceutical industry and are found in virtually all regulatory filings.
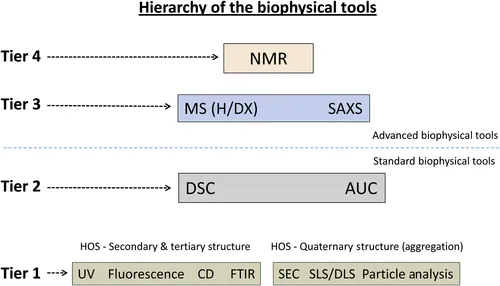
In the second grouping, only biophysical tools in tier 3 and 4 have been included. Tools in this grouping consist of what we call the more “advanced” biophysical tools. Although these tools are presently used in a limited capacity in the process development area, we anticipate their utility will expand in the coming years. Their ability to generate information with greater detail and higher resolution will help improve the industry's ability to characterize biopharmaceuticals to make better and sounder decisions concerning a range of critical evaluations. This should facilitate improvements in the overall biopharmaceutical development process by increasing the success rate of drug approvals, while at the same time reduce the cost and the time required in their development.
4.2. The Standard Class of Biophysical Tools Used in the Biopharmaceutical Industry
The biophysical tools listed in this category correspond to the standard set of tools used throughout the development process of most biopharmaceuticals. Nearly all of these tools will be employed in various phases of development work from comparability and stability studies to formulation development. In addition, they will likely be represented in characterization sections in investigational new drug and biological license application filings under the heading of biophysical or physicochemical characterization. In particular, size-exclusion chromatography (SEC) and particle analysis methods will, in most cases, be developed into lot release assays where they will be used to monitor aggregation. In the case of particle analysis, it is very likely this area will consist of more than one specific test (see Chapter 10). In addition to direct visual testing, obtaining information about subvisible and submicron particle analysis is becoming critical...
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- List of Contributors
- About the Editors
- Preface
- List of Abbreviations and Symbols
- I. Proteins and Biophysical Characterization in the Biopharmaceutical Industry
- II. The Selected Biophysical Tools in the Biopharmaceutical Industry
- III. Concluding Remarks on the Biophysical Characterization of Biopharmaceuticals
- Index