
- 452 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Carbohydrates
About this book
There is a vast and often bewildering array of synthetic methods and reagents available to organic chemists today. The Best Synthetic Methods series allows the practising synthetic chemist to choose between all the alternatives and assess their real advantages and limitations.
Each chapter in Carbohydrates details a particular theme associated with carbohydrate synthesis. A brief review of the subject area is provided, but the emphasis in all cases is on describing efficient practical methods to effect the transformations described.
In order for the roles of carbohydrates to be thoroughly analysed and assessed, glycobiologists require access to defined target carbohydrates in useful quantities. Thus carbohydrates and glycoconjugates are now recognized as important targets for total synthesis programmes and it is essential to develop efficient regio- and stereoselective methods for the synthesis of carbohydrates. Whilst carbohydrates can sometimes be isolated from natural sources, synthetic strategies often offer the advantage of allowing access to larger quantities of material as well as entry to analogues of the natural carbohydrates.
- The latest volume in the long standing Best Synthetic Methods series
- Clear chapter by chapter breakdown of carbohydrate synthesis themes with examples of good practical methods for common carbohydrate syntheses
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
Physical SciencesSubtopic
Organic Chemistry1 An Introduction to Carbohydrate Synthesis
1.1 BIOLOGICAL ROLES OF CARBOHYDRATES
In the past, carbohydrates were considered to be solely of use for energy storage, and as skeletal components. However, this naïve view was challenged in 1963 when a protein was isolated from Canavalia ensiformis that demonstrated ability to bind to carbohydrates on erythrocytes. In 1982 the first animal carbohydrate binding protein was identified, and this sparked interest into the wider roles of carbohydrates and carbohydrate binding proteins within biological systems. These carbohydrate binding proteins are termed lectins and it is now known that they are found in varying densities on all cell-surface membranes [1]. They interact specifically with oligosaccharides and glycoconjugates (such as glycolipids and glycoproteins) on the surrounding cells via hydrogen bonding, metal coordination, van der Waals forces, and hydrophobic interactions. It is believed that favourable interactions occur between the hydroxyl groups of the carbohydrates and the amino acid functionalities of the proteins, to aid molecular recognition processes. These interactions are relatively weak, but they are so numerous that specific interactions occur. Selectivity is believed to be further increased through additional binding of the carbohydrate to the lectin’s subsites.
The study of carbohydrates within biological systems has illustrated that they are involved in a number of fundamental biological functions such as cell–cell recognition and cell-external agent interactions [2]. These interactions can initiate beneficial biological events [2], such as fertilization, cell growth and differentiation (for example during embryogenesis) [3] and immune responses, as well as detrimental disease processes [2], such as inflammation, viral and bacterial infections, and cancer metastasis (vide infra). Carbohydrates of even short sequences are used for carrying biological information, for example, the human blood groups are differentiated by relatively simple changes in oligosaccharide structure (Figure 1.1).
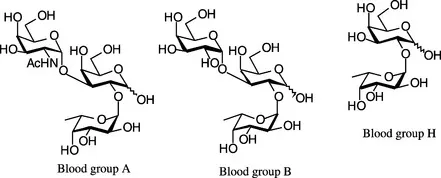
Figure 1.1
Therefore the view that carbohydrates are of limited importance within biological systems has been challenged and renewed interest into the science of ‘Glycobiology’ has emerged.
In order for the roles of carbohydrates to be thoroughly analysed and assessed, glycobiologists require access to defined target carbohydrates in useful quantities. Thus carbohydrates and glycoconjugates are now recognized as important targets for total synthesis programmes. If access to biologically important carbohydrates can be achieved, then material will be available for a number of means:
(1) Some bacterial surface proteins demonstrate specific binding for carbohydrates expressed on human cells, and such interactions form an essential part of the infection pathway. It has been demonstrated that administration of synthetic or natural carbohydrate derivatives can disrupt this infective pathway, so long as the administered derivatives have a high affinity for the bacterial lectins [4]. In such cases, the bacteria are no longer able to interact with the host, and therefore pass through the body without initiating infection. Such therapeutic agents have been termed anti-infective agents. A number of anti-infective agents occur naturally, for example, human breast milk contains numerous soluble oligosaccharides that provide newborn babies with a mechanism for aborting infection processes (Figure 1.2) [5].
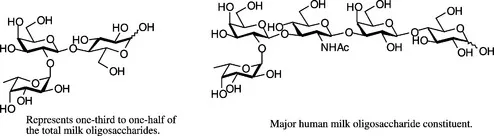
Figure 1.2
An alternative approach for treating bacterial infections has seen the development of carbohydrate based antibiotics to target carbohydrate receptors and carbohydrate modifying enzymes [6].
(2) The synthesis of disease associated carbohydrates may hold the key for the development of vaccination strategies for the respective diseases [7]. For example, the use of tumour associated carbohydrate antigens for raising antibodies for the treatment of cancer is currently being developed (Figure 1.3) [8].
(3) The synthesis of carbohydrate analogues is also being pursued in an attempt to inhibit the interactions between the carbohydrates and selectins that are essential for disease progression [9]. This has received particular attention for inhibiting tumour growth and metastasis. The natural role of the selectins is to assist the ‘rolling’ of leukocytes on the surface of activated endothelial cells in the blood vessels. However, the unusual carbohydrates on cancer cells also provide tumour cells with a mechanism for moving along the endothelial cells, in a process known as metastasis [10]. Therefore novel cancer treatments are investigating the possibility of utilizing soluble carbohydrates to block the selectin sites on the epithelial cells in the blood vessels to inhibit metastasis.
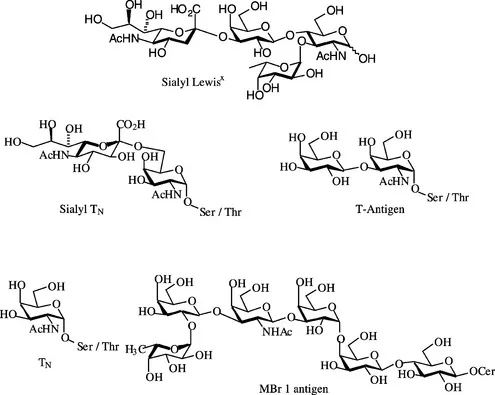
Figure 1.3
An alternative approach has concentrated on aborting the synthesis of disease associated carbohydrates, and this again requires access to carbohydrate analogues [11]. The assembly of carbohydrates within biological systems occurs in the endoplasmic reticulum of the golgi apparatus and involves a number of enzyme mediated steps [12]. Biosynthesis commences with the formation of a Glc3Man9GlcNAc2 oligosaccharide which is covalently bound to a lipid. This is transferred from the lipid to a peptide via a further enzyme and a series of glycosyl hydrolase and glycosyl transferase enzymes then proces...
Table of contents
- Cover image
- Title page
- Table of Contents
- BEST SYNTHETIC METHODS
- Copyright
- Dedication
- Acknowledgements
- Contributors to this Volume
- Abbreviations
- Chapter 1: An Introduction to Carbohydrate Synthesis
- Chapter 2: Selective Hydroxyl Protection and Deprotection
- Chapter 3: Synthesis and Activation of Carbohydrate Donors: Acetates, Halides, Phenyl selenides and Glycals
- Chapter 4: Synthesis and Activation of Carbohydrate Donors: Thioglycosides and Sulfoxides
- Chapter 5: Synthesis and Activation of Carbohydrate Donors: Acetimidates, n-Pentenyl and Vinyl Glycosides
- Chapter 6: Modern Glycosidation Methods: Tuning of Reactivity
- Chapter 7: Modern Glycosidation Methods: Orthogonal Glycosidation
- Chapter 8: The Stereoselective Synthesis of β-Mannosides
- Chapter 9: Synthesis of Sialic Acid Containing Carbohydrates
- Chapter 10: The Synthesis of Glycosyl Amino Acids
- Chapter 11: The Synthesis of C-linked Glycosides
- Chapter 12: The Uses of Glycoprocessing Enzymes in Synthesis
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Carbohydrates by Helen Osborn in PDF and/or ePUB format, as well as other popular books in Physical Sciences & Organic Chemistry. We have over one million books available in our catalogue for you to explore.