
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Until the early 1960s, cancer treatment consisted primarily of surgery and radiation therapy. Most practitioners then viewed the treatment of terminally ill cancer patients with heroic courses of chemotherapy as highly questionable. The randomized clinical trials that today sustain modern oncology were relatively rare and prompted stiff opposition from physicians, who were loath to assign patients randomly to competing treatments. Yet today these trials form the basis of medical oncology. How did such a spectacular change occur? How did medical oncology pivot from a nonentity and, in some regards, a reviled practice to the central position it now occupies in modern medicine?
In Cancer on Trial Peter Keating and Alberto Cambrosio explore how practitioners established a new style of practice, at the center of which lies the cancer clinical trial. Far from mere testing devices, these trials have become full-fledged experiments that have redefined the practices of clinicians, statisticians, and biologists. Keating and Cambrosio investigate these trials and how they have changed since the 1960s, all the while demonstrating their significant impact on the progression of oncology. A novel look at the institution of clinical cancer research and therapy, this book will be warmly welcomed by historians, sociologists, and anthropologists of science and medicine, as well as clinicians and researchers in the cancer field.
In Cancer on Trial Peter Keating and Alberto Cambrosio explore how practitioners established a new style of practice, at the center of which lies the cancer clinical trial. Far from mere testing devices, these trials have become full-fledged experiments that have redefined the practices of clinicians, statisticians, and biologists. Keating and Cambrosio investigate these trials and how they have changed since the 1960s, all the while demonstrating their significant impact on the progression of oncology. A novel look at the institution of clinical cancer research and therapy, this book will be warmly welcomed by historians, sociologists, and anthropologists of science and medicine, as well as clinicians and researchers in the cancer field.
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription.
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn more here.
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 1000+ topics, we’ve got you covered! Learn more here.
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more here.
Yes! You can use the Perlego app on both iOS or Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Yes, you can access Cancer on Trial by Peter Keating,Alberto Cambrosio in PDF and/or ePUB format, as well as other popular books in Medicine & Medical Theory, Practice & Reference. We have over one million books available in our catalogue for you to explore.
Information
PART 1
The Emergence of Clinical Cancer
Research (1955–66)
Research (1955–66)
CHAPTER TWO
A Landmark Clinical Trial
Curing Leukemia: The VAMP Trial
In the summer of 1961, two members of the NIH Clinical Center in Bethesda (Maryland), Emil Frei, the chief of the Medicine Branch at the NCI, and Emil J Freireich, a senior investigator in the same branch, decided to conduct a clinical trial to test a combination of anticancer drugs on children afflicted with acute lymphocytic leukemia (ALL). They launched the study, whose protocol they summarized under the evocative rubric VAMP, in early 1962. The results were so promising that they stopped the trial after treating a mere sixteen children. As a consequence and despite the subsequent notoriety of the protocol—considered the first combination of drugs to actually “cure” childhood leukemia—the only published trace of the original plan remains a brief abstract in the Proceedings of the American Association for Cancer Research (Freireich, Karon & Frei, 1964).
As described in the Proceedings, the protocol set out a rigorous regime of chemotherapy that combined four different substances. Frei and Freireich had chosen the drugs so that each had a different mechanism of action. Each agent thus destroyed cancer cells in a different way, creating, the researchers hoped, a cumulative, or even synergistic, effect. The protocol also stipulated different routes of administration and schedules for each drug: vincristine (V) and amethopterin (A, also called methotrexate) were to be given every four days intravenously; mercaptopurine (M) and prednisone (P) were to be administered orally on a daily basis. The protocol further specified that the treatment would continue until a patient achieved “remission,” defined not only as the abatement of the leukemic symptoms but also as the reduction of the proportion of leukemic cells in the bone marrow to below 5% of the total number of cells contained therein. Once in remission, the protocol then submitted the patients to five more ten-day courses. A “rest period” that lasted either ten days or until toxicity subsided followed each course. In case of relapse following remission, treatment began anew.
Frei and Freireich used the amount of time the young patients spent in complete remission as the end point of the trial. When the trial finished, the median remission time stood at 150 days. To present-day readers, the decision to stop the trial may appear somewhat surprising given the small numbers. The sixteen “consecutive” patients—the first sixteen children with acute leukemia to come through the doors of the NIH Clinical Center—were the only patients studied. So why stop so soon after beginning? The simple answer is: 150 days was the longest remission yet achieved in the treatment of early childhood leukemia by either the study trialists or by others in the field.
The others in the field included members of several collective undertakings known as Cooperative Groups. In addition to being researchers at the NIH Center, Frei and Freireich also belonged to a research collective that conducted clinical cancer trials in leukemia known as the Acute Leukemia Group B (ALGB). Along with other members of that collective, Frei and Freireich had undertaken previous trials with each of the substances in VAMP. The group had thus developed what are often termed “historical controls,” and Frei and Freireich were consequently able to compare the results of VAMP with the results obtained in the earlier trials. In the case of prednisone, for example, a trial completed the year before and known as “Protocol No. 3” had produced only sixty-day remissions (Frei & Freireich, 1965: 284). The remissions produced by VAMP, however, were not simply better than prior results: both the VAMP results and the results of the prior studies were all quite unprecedented.
Each of the drugs used in VAMP had been discovered in the postwar era, and three of the four had been developed in the previous decade. We have already discussed the oldest member of the combination, methotrexate. One of the more recent compounds, vincristine, was the first naturally occurring substance to be used as an anticancer agent. Derived from an extract of the Madagascar periwinkle, a plant used as a folk remedy for centuries, it had long been reputed to be a useful remedy in diabetes (Noble, 1990). Following up on these folk reports in a search for an oral compound to treat diabetes, the American pharmaceutical giant Eli Lily had begun examining extracts of the leaves of the periwinkle in the 1950s and discovered that they contained over seventy different alkaloids (Pearce & Miller, 2005). Canadian researchers had embarked on a similar quest but for logistic reasons were unable to compete with Eli Lily; to extract one ounce of the drug, researchers had to process fifteen tons of leaves. So even though the Canadians had enrolled Jamaican Boy Scouts to collect the leaves, they were in the end forced to rely on extracts produced by Eli Lily. Early tests of the extracts had shown, unexpectedly, anticancer properties. Thus despite the initial interest in an insulin substitute, the Canadians went on to perform the first successful human cancer tests in the late 1950s (Duffin, 2002a; 2002b) and subsequently forwarded the substance to the NCI for further examination (Carbone et al., 1963).
While the discovery of the anticancer properties of vincristine was partly serendipitous, prednisone emerged in 1955 following intensive research in the field of steroid chemistry that had begun with the isolation of the sex hormones in the 1920s. By the time of prednisone’s synthesis, greatly stimulated by the discovery of an extremely lucrative and structurally similar compound, cortisone, in 1948, all the major pharmaceutical companies had established research programs in the field of steroids. Although Schering ultimately won the patent for prednisone in 1964, five other pharmaceutical companies (Merck, Squibb, Pfizer, Upjohn, and Syntex) also claimed priority for the drug in what was clearly a case of multiple discovery (Herzog & Oliveto, 1992; Heusler & Kalvoda, 1996; Hillier, 2007).
When prednisone entered the cancer research market as an experimental substance in the mid-1950s, it was not the only steroid in use. A similar hormone, ACTH, isolated in 1949, had already shown some activity in ALL. Prednisone’s career as an anticancer agent thus began as a substitute for a known agent and initially did little to distinguish itself. As Sidney Farber commented in 1956 when reviewing cortisone, ACTH, prednisone, and fluorohydrocortisone: “In their effect, all agents are similar and may be discussed as one” (Farber et al., 1956: 13). Those effects were distressingly minimal. None produced remissions that lasted longer than seventy days and all had a tendency to produce “Cushing’s syndrome,” whose principal symptoms—weight gain, thinning of the skin, depression, and anxiety—merely added to the growing list of unpleasant side effects produced by anticancer compounds.
The fourth and final component of the VAMP combination, 6-mercaptopurine (6-MP), was also a product of systematic industrial research and earned its discoverers, Gertrude Elion and George Hitchings, the 1988 Nobel Prize. The two Wellcome Company researchers had begun the work leading to the discovery of 6-MP in the early 1950s in a search for compounds to block bacterial reproduction. They had found that bacteria could not reproduce without a class of compounds known as purines. They knew purines were among the building blocks of DNA (well before the discovery of the structure of DNA in 1953 by Watson and Crick) and consequently began hunting for compounds to interrupt purine production. 6-MP emerged in the course of this research. Early animal tests of the compound showed that it inhibited the growth of a variety of rat and mouse tumors, and by 1953, 6-MP had induced shortlived remissions in humans (Quirke, 2009).
Within a relatively short period then, industrial researchers had developed three different compounds that produced regressions in childhood leukemia. By themselves, none of the substances produced longlasting remissions or anything that could be even remotely associated with a cure. Moreover, even when used sequentially or two at a time, although remissions were accelerated, all patients died eventually as resistant forms of leukemia emerged to defeat the drugs. Low expectations thus replaced the initial excitement that had surrounded the drugs. As one of the pioneers of leukemia therapy in the 1960s has noted, the failure of the anticancer compounds in the 1950s “led to a fixed notion among most pediatricians and hematologists that temporary remissions and prolongation of survival in comfort were the most one could expect from leukemia chemotherapy” (Pinkel, 2006: 12).
Two ancillary components of the VAMP protocol that remain crucial to the cure of acute leukemia in children were filtered air and the frequent transfusion of blood platelets. The transfusions combated the severe bleeding in acute leukemia patients that results from a loss of cells responsible for blood clotting. Provoked by the chemotherapy, the bleeding was sometimes so severe that it often became the immediate cause of death. One of the conditions of possibility for treating leukemia patients with chemotherapeutic substances was thus the control of bleeding so that the patients might live long enough for the drugs to have an effect.
Freireich and his colleagues had developed the blood platelet transfusion techniques just before the VAMP trial and had done so in part at the behest of the head of the chemotherapy program at the NCI, Dr. Gordon Zubrod. As Freireich recalled:
I was in charge of the leukemia program and Frei ran the solid tumor and Dr. Zubrod was the godfather and he’d come on rounds every once in a while. They’d come on the leukemia rounds, and in the leukemia ward with these children there was blood all over. There was blood on the sheets, blood on the pillows, blood on the floor; the nurses were covered with blood…. Zubrod came out in the hall one day and said, “This is a bloody ugly mess, Freireich. Why don’t you do something about hemorrhage?” So being that I was a young guy, respectful, I said, “Yes sir.” I went to the lab and I did simple experiments…. If you just take the children’s plasma and take fresh platelets in the laboratory, it is 100% corrected. So I said, gee-whiz, all we got to do is give them platelets.1
The process was slightly more complicated than Freireich’s recollections suggest. When the NCI Clinical Center researchers began their work on the control of bleeding, whole blood transfusions had generated some controversy since “classical hematologists had supposedly shown, using labeled (and therefore damaged) platelets, that the infused platelets did not last more than a few hours” (Zubrod, 1984: 11). So Freireich and Frei first had to undo this doctrine. Moreover, while fresh blood transfusions raised platelet levels more significantly than blood drawn from blood banks, neither method seemed adequate because of the sheer amount of blood that had to be transfused into patients (Freireich et al., 1959). To overcome the blood shortage problem and to limit the size of the therapeutic transfusions, Frei and Freireich sought ways to concentrate platelets and to calculate the minimum number of platelets necessary to stop bleeding (Gaydos, Freireich, & Mantel, 1962). This initiative also proved controversial:
These studies were vigorously attacked by classical hematologists because they required larger amounts of blood than blood banks could easily obtain. I recall a dramatic showdown at a clinical staff meeting in 1956 where a motion was made to deny platelets to the NCI. In those days the entire clinical staff of the Clinical Center met monthly to discuss patient care problems. The other clinical directors came to our support and the motion was narrowly defeated. (Zubrod, 1984: 11)
In collaboration with members of the Division of Biological Standards at the NCI, Freireich developed a device that would allow them to take blood from donors, separate the platelets, and return the red blood cells to the donor, thus creating the possibility of multiple donations from a single donor (Kliman et al., 1961). While the platelet concentration system worked well enough for the VAMP patients, it was labor intensive and the search for a large-scale source of platelets became an ongoing concern both for Freireich, who continued working on the problem for the next twenty years, and other members of ALGB who pursued cooperative research with IBM to produce an automatic cell separator (Hester et al., 1985).2
The chemotherapy itself created further problems. Leukemia patients submitted to the rigors of methotrexate, 6-MP, and prednisone lost other components of their blood, most notably the white blood cells, and as a result suffered severe immunodeficiency and thus frequent infection. Like other researchers, Frei and Freireich treated the problem of infection through megadoses of intravenous antibiotics. They also resorted to other devices such as oxygen tents when, in spite of precautions, the children caught pneumonia. But even that was not enough, and so they went one step further: in order to combat antibiotic-resistant fungal infections caused by common inhabitants of hospital air ducts, the NCI researchers developed “life islands” equipped with special air filters (figure 2.1) (Nathan, 2007). In recognition of the importance of these “ancillary measures,” Life magazine chose to illustrate its 1966 report on the “all-out assault on leukemia” with photographs showing not only bottles of anticancer drugs but also the accompanying infrastructure (figure 2.2).
When all was said and done, compared with the disappointments of the 1950s, the results of the VAMP protocol were quite spectacular. Of the sixteen patients treated, thirteen achieved “complete remission.” More importantly, the time spent in remission lasted two to three times longer than any remission obtained using a single compound or any combination of two compounds. Finally, when Frei and Freireich reported their results in a review article (1965), two of the patients were still alive and in complete remission after over six hundred days, a result heretofore unseen.
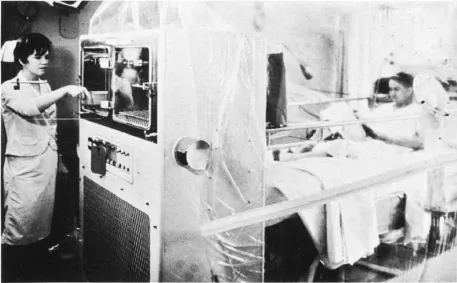
FIGURE 2.1. A “life island.” Reprinted with kind permission from G. P. Bodey, P. Watson, C. Cooper & E. J. Freireich, “Protected Environment Units for the Cancer Patient,” CA: A Cancer Journal for Clinicians 21 (1971): 216. © 1971, John Wiley and Sons.
The VAMP trial illustrates three important aspects of the early development of chemotherapy. The first is that contrary to the unrealistic expectations maintained in some quarters, successful chemotherapy was not produced by “magic bullets.” For while some chemotherapists of the 1940s and early 1950s entertained the possibility of finding a drug or a category of drugs that, as antibiotics did for bacterial infections, would wipe out cancer cells with no important side effects, cooperative group trialists quickly showed such fancies to be counterproductive. They noted, in particular, that “prospecting for the ‘penicillin’ of cancer [would be] wasteful of the opportunity to detect lesser degrees of activity and to validate or improve [the trialists’] abilities to select more useful compounds.”3 And indeed, therein lay yet another important lesson of the VAMP trial: not only were the prospective magic bullets less than supernatural, they also produced significant collateral damage. As a result, in order to transform chemotherapy into a viable option, an entire infrastructure—ranging from mechanical devices to other pharmacological substances—had to be put in place. The deployment and assessment of (candidate) chemotherapeutic compounds thus became dependent upon this infrastructure.

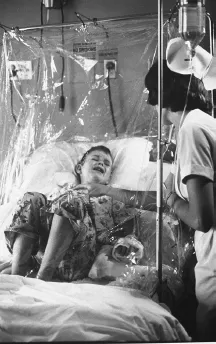
FIGURE 2.2. In an abundantly illustrated report titled “The All-Out Assault on Leukemia” and focusing on a young patient named Mike Parker, Life magazine highlighted both anticancer drugs and the hospital infrastructure necessary to turn those drugs into a viable option. (a) The headline above the picture reads: “Of 170,000 drugs tested, eight might help Mike Parker”; the eight drugs are in the foreground, and 6MP is the fifth from left. (b) Mike Parker’s immune system having been weakened by anticancer drugs, an oxygen tent is used to fight pneumonia. Reproduced with kind permission from W. Bradbury, “The All-Out Assault on Leukemia: Two Views—the Lab, the Victim,” Life, 16 November 1966; (a) pp. 92–93, © Fritz Goro/Time & Life Pictures/Getty Images; (b) p. 104, © Leonard McCombe/Time & Life Pictures/Getty Images.
A second related aspect of the trial reminds us that, as noted in chapter 1, early chemotherapy was a “heroic” endeavor that some physicians regarded as not quite legitimate: a therapeutic delusion that far from curing or at least significantly prolonging the life of patients resulted in unnecessary suffering. VAMP, because it induced lasting remissions and incorporated strategies to tame the side effects of chemotherapy, represented a de facto rebuttal of this criticism. Last but not least, the “polychemotherapy” ...
Table of contents
- Cover
- Copyright
- Title Page
- Dedication
- Contents
- Foreword
- Acknowledgments
- INTRODUCTION
- PART 1. The Emergence of Clinical Cancer Research (1955–66)
- PART 2. An Avalanche of Numbers from the New Style of Practice (1965–89)
- PART 3. Targeted Therapy, Targeted Trials (1990–2006)
- CONCLUSION
- Notes
- References
- Index