
- 414 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Cellular Endocrinology in Health and Disease
About this book
Cellular Endocrinology in Health and Disease describes the underlying basis of endocrine function, providing an important tool to understand the fundamentals of endocrine diseases. Delivering a comprehensive review of the basic science of endocrinology, from cell biology to human disease, this work explores and dissects the function of a number of cellular systems. Among these are those whose function was not obvious until recently, including the endocrine functions of bone and the adipose tissue.Providing content that crosses disciplines, Cellular Endocrinology in Health and Disease details how cellular endocrine function contributes to system physiology and mediates endocrine disorders. A methods section proves novel and useful approaches across research focus that will be attractive to medical students, residents, and specialists in the field of endocrinology, as well as to those interested in cellular regulation. Editors Alfredo Ulloa-Aguirre and P. Michael Conn, experts in molecular and cellular aspects of endocrinology, deliver contributions carefully selected for relevance, impact, and clarity of expression from leading field experts.- Covers systemic endocrine action at the cellular level in both health and disease- Delivers information on the integration of cell identity and endocrinology- Incorporates recent developments in endocrinology to provide an up-to-date reference to researchers
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
Thyroid Hormone Receptors and their Role in Cell Proliferation and Cancer
Keywords
Thyroid Hormone Action
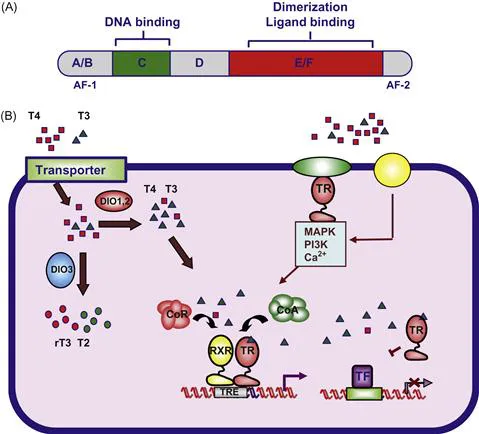
(A) Schematic representation of a thyroid hormone receptor, showing the different functional domains. (B) Thyroxine (T4) and triiodothyronine (T3) enter the cell through transporter proteins such as MCT8 and 10 or OATPs. Inside the cells, deiodinases (DIO1,2) convert T4, to the more active form T3. DIO3 produces rT3 and T2 from T4 and T3, respectively. T3 binds to nuclear thyroid hormone receptors (TRs) that regulate transcription by binding, generally as heterodimers with the retinoid X receptor (RXR), to positive or negative thyroid hormone response elements (TREs) located in regulatory regions of target genes. Activity is regulated by an exchange of corepressor (CoR) and coactivator (CoA) complexes. TRs can also regulate the activity of genes that do not contain a TRE through “cross-talk” with other transcription factors (TF) that stimulate target gene expression. Binding of T3 to a subpopulation of receptors located outside the nuclei can also cause rapid “non-genomic” effects through interaction with adaptor proteins, leading to stimulation of signaling pathways. T4 can also bind to putative membrane receptors such as integrin αVβ3 inducing mitogen activated protein kinase (MAPK) activity.
TRs and Cancer
Table of contents
- Cover image
- Title page
- Table of Contents
- Copyright
- List of Contributors
- Preface
- Chapter 1. Thyroid Hormone Receptors and their Role in Cell Proliferation and Cancer
- Chapter 2. The Molecular Cell Biology of Anterior Pituitary Cells
- Chapter 3. Sensing Calcium Levels: The Biology of the Parathyroid Cell
- Chapter 4. The Biology of Pituitary Stem Cells
- Chapter 5. The Na+/I− Symporter (NIS) and Thyroid Hormone Biosynthesis
- Chapter 6. The Follicle-Stimulating Hormone Signaling Network in Sertoli Cells
- Chapter 7. Epigenetics of Pituitary Cell Growth and Survival
- Chapter 8. Epigenetic and Developmental Basis of Risk of Obesity and Metabolic Disease
- Chapter 9. Unraveling the Mechanism of Action of the GnRH Pulse Generator: A Possible Role for Kisspeptin/Neurokinin B/Dynorphin (KNDy) Neurons
- Chapter 10. Proteomics in Reproduction: The Dialogue Between the Blastocyst and the Endometrium
- Chapter 11. Transcriptome Analysis of Adrenocortical Cells in Health and Disease
- Chapter 12. Bone as an Endocrine Organ
- Chapter 13. Regulation of Steroidogenesis
- Chapter 14. Adipose Tissue as an Endocrine Organ
- Chapter 15. Insulin-Secreting Cell Lines: Potential for Research and Diabetes Therapy
- Chapter 16. Architecture and Morphology of Human Pancreatic Islets
- Chapter 17. Computational Models to Decipher Cell-Signaling Pathways
- Chapter 18. Defects in Ovarian Steroid Hormone Biosynthesis
- Chapter 19. Control of the GnRH Pulse Generator
- Chapter 20. Endocrinology of the Single Cell: Tools and Insights
- Chapter 21. Intracellular Trafficking of G Protein-Coupled Receptors to the Plasma Membrane in Health and Disease
- Chapter 22. Iodothyronine Deiodinases: Emerging Clinical Crossroads
- Chapter 23. MicroRNAs and Long Non-Coding RNAs in Pancreatic Beta Cell Function
- Index