![]()
— 1 — Inside evolution
A new look at evolution
IT’S another cloudy, windswept day. The wet streets are glistening. You quicken your pace as the rain starts coming down harder. Just a few hundred metres to go and then you’ll be there. A car speeds past and sends a spray of muddy water over you. Will it really be worth it? But then, what better thing to do on such a day but visit the museum. After all, it’s one of the few places of free entertainment left. Entertainment? Not much in this one. The museum in this small country town has seen better days.
You round the corner and there it is—the red-brick mausoleum, resting place of so many dead bodies, and decaying feelings of the past. But you rush up the steps to get out of the rain. The difference is stunning. The roar and hiss of passing vehicles and the clatter and the noise of the town are suddenly gone. Here all is dry; all is dust. The only sound is the dripping of water off your coat on to the cold floor, and the buzzing of the lamps. The dull yellow lights spread a pale, sickly pallor over the stuffed, moth-eaten bear that greets you. The smell is the smell of museums. There is no other way to describe it. But not one to bottle and cherish. Once smelt it’s never forgotten.
In to the small room to your left, past the flint tools, lovingly crafted by some distant soul too many thousands of years ago to even contemplate. Past the brown chipped pots and the faded images of cavemen huddled round a fire. And then you find it. Tucked away at the back of the room in a glass case is the epitome of evolution—plaster casts of bare bones from creatures said to be horses. But these were strange horses, sporting more than one toe and big enough only for a vervet monkey to ride on. Many museums around the world have, at one time or another, had just such a display case. Thankfully many are now gone, but some still remain, purporting to show the classic example of evolution of the horse—a simple evolutionary trend from a four-toed, tiny horse to the massive creature that today imperiously snorts at you from its exalted height.
Usually labelled as Eohippus, this first ancient horse, that scurried through the woodlands of North America 55 million years ago is now called Hyracotherium. The label in the display case tells of how this horse evolved into Mesohippus, then into Mercyhippus, then Pliohippus in more recent times, before being replaced by the mighty Equus, winner of Melbourne Cups and Grand Nationals. All this in a little over 50 million years. And as the horses got bigger, their teeth became more complex, allowing the later, larger horses to move out into the newly evolving green pastures, to graze, rather than to browse, and to gallop down the home straight not on legs with four toes, but on legs sprouting just one mighty hoof. The trouble with this lovely story is that it is a gross oversimplification. Evolution of such a major group of animals is not like an arrow hurtling through time. It is a complex branch with many dead ends and hopeless causes, successes and failures, and being in the right pace at the right time, and avoiding being eaten, or. becoming extinct. If you survive these hurdles you might be one of the winners in the evolutionary handicap.
We often view evolutionary change purely in terms of what adaptive advantage it bestowed upon its descendants, without trying to unravel the underlying processes that actually cause these changes to occur. Examples like the evolution of horses, when interpreted in terms of modern evolutionary theory, allow the fossil record to be a major contributor to our understanding of both the patterns and the processes, of evolution. However, our perception of how organisms change through time has been blinkered by a concentration on looking purely at adult characters. I’m sure you’ve seen the sort of illustration—humans evolving from some hairy ape into a dapper businessman, and our horses evolving from tiny animals the size of a fox to a massive shire horse—always depicted in terms of changes to the adult. Yet natural selection is not all that selective. It is weaving its spell from the moment of conception, picking and choosing between different organisms, or their parts, as the organism undergoes its embryonic or larval development, through its childhood until it is an adult, for most organisms change in appearance appreciably as they grow up.
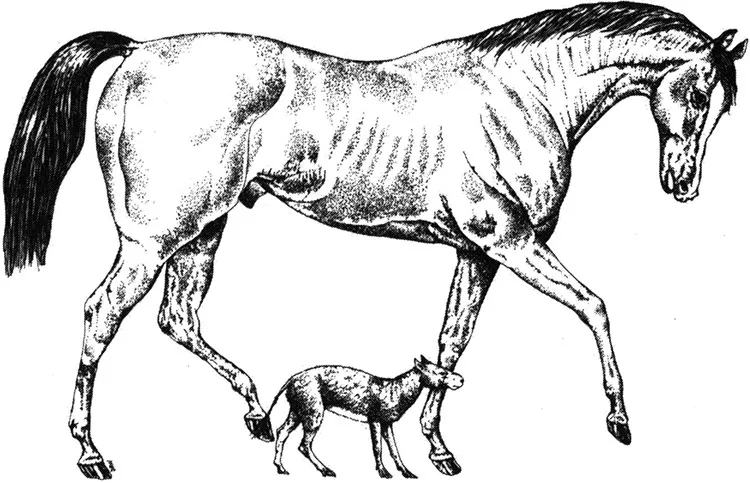
A horse is a horse, of course of course. Or is it? Hyracotherium, the first primitive horse about the size of a fox terrier, scampers beneath a modern racehorse, that well-known triple Derby winner, ‘Stillrunning’ (genus Equus).
Evolution is largely about tinkering with what is already there. Massive genetic mutations are not necessary in order to evolve new shapes, new sizes and new behaviours. What is important in evolution are changes to the rate and timing of development—how fast an organism grows during its development, compared with its ancestor, and for how long. This is known as ‘heterochrony’ (literally different times). Essentially, all that is happening in heterochrony is that an animal or plant passes through more or fewer growth and development stages than its ancestor. If fewer stages are passed through it is called ‘paedomorphosis’—meaning ‘child shape’. If it goes through more it is called ‘peramorphosis’, meaning ‘beyond shape’. Paedomorphosis can arise from a slower growth rate, finishing growth earlier (for example, sexual maturity might arrive earlier) or starting growth later. Peramorphosis, on the other hand, can arise from faster growth, or from growing for a longer period, by delaying the offset of growth or turning it on earlier. While some species may show just one type or another, being either paedomorphic or peramorphic, many are a mosaic of both features.
So, what about horses? There is no escaping the fact that early horses were much smaller than modern horses. But rather than a progressive increase in body size over more than 50 million years, what occurred was an increase in the diversity of body size. As well as the evolution of new large species through the Cenozoic era, new small horses evolved. Indeed, up until about 10 million years ago there were species of horses that still weighed only about 75 kg, although others weighed in at 400 kg. Such size increase that did occur would have happened by either an acceleration in the rate of growth or an extension of the period of growth—or both. In other words, a delay in the onset of maturity allows the period of rapid juvenile growth to carry on for longer. Direct evidence exists from the fossil record to indicate that Miocene horses, for example, living about 20 million years ago, reached sexual maturity at an earlier age than later horses. Extending the period of growth in later horses produced not only a larger animal, but also changes in shape of many parts of the body.
As organisms grow, different parts grow at different rates with respect to each other, and may themselves vary their growth rates during development. This results in distinct changes in shape during development. Dogs are a classic example of this. Compare the newborn puppy of a german shepherd with the adult, and there are some substantial differences. The puppy has a much larger head, relative to the rest of the body. Its limbs, on the other hand, are proportionately smaller. Its face is flatter, and during development the muzzle increases greatly in size and changes substantially in shape. This pattern holds, to varying degrees, for many mammals. As a result, changing the rates or periods of growth can induce substantial morphological changes.
Natural selection may target particular morphological traits. Often one trait develops seemingly at the expense of others—a developmental trade-off An example of this is the classic evolutionary change in the number of horse toes. Through a range of species there was an overall trend for a reduction in the number of toes in the fore and hindlimbs. This occurred by a reduction in size—probably brought on by a reduction of the growth rate—of each of the toes, apart from one. This toe, on the other hand, underwent an increase in the amount of its growth, either an acceleration or an increase brought about simply by the overall increase in body size, dragging this single toe along with it to become the hoof. A similar event happened in the evolution of the extinct sthenurine kangaroos. They likewise ended up with a solitary toe—racehorses of the kangaroo world. South American litopterns, which evolved some 50 million years ago, were rather horse-like in appearance, and they too independently underwent a reduction in toe number, from three to one in forms living 20 million years ago. In all these cases we have more examples of classic developmental trade-offs—reduction in the number of toes (paedomorphosis), but a great increase (peramorphosis) in the remaining one to produce a hoof—essentially just an enormous toenail.
Another way that heterochrony operates is by stretching out successive developmental periods. It is quite possible that this process is also responsible for perhaps the most classic example of paedomorphosis seen among living animals—the Mexican axolotl, Ambystoma mexicanum. Long touted as a classic example of ‘neoteny’, which is a reduction in the rate of growth, the appearance of the axolotl—looking for all the world like a juvenile salamander, yet being sexually mature—does not arise from a change to its rate of growth. Whereas many salamanders undergo a metamorphosis from an aquatic juvenile form, which has gills, to a land-based, air-breathing pre-adult form, which then matures on land to become a land-based adult, the axolotl remains aquatic throughout its life. Consequently, as a sexually mature adult it retains the premetamorphic juvenile head, complete with external gill anatomy. To understand how this comes about we have to look not at how fast it grows, but at the timing of transformation, or lack of transformation, from one developmental stage to another.
The ancestral developmental sequence, such as occurs in a related species Ambystoma tigrinum passes through embryonic, larval, metamorphic and postmetamorphic periods. Following metamorphosis, the salamander leaves the water to live on land. The axolotl follows the same ancestral embryonic and larval developmental pattern to a point when the head undergoes no further shape change. It fails to undergo metamorphosis—which the ancestral form does when about 6 months old—instead remaining, Peter Pan-like, in a state of perpetual youth. There is no difference in the time at which sexual maturity is initiated in either species, both occurring at about 10 months. What is happening is that growth and development of the body is quite dissociated from gonadal development, which determines when the species becomes sexually mature. Because the axolotl fails to metamorphose, the ancestral premetamorphic larval features are retained by the descendant adult, so it remains living in an aquatic environment.
Evolution through taking ugly pills? No, not really, just that beauty is always in the eye of the beholder, especially if you were a brontothere. Progressive large size with increasing horn development was seen as a natural trend in the evolution of several groups of mammals arising from the stretching out of periods of growth and development.
This same phenomenon can actually occur within some species of salamanders. Stephen Reilly of Ohio University found that in the living mole salamander Ambystoma talpoideum metamorphosis normally occurs during the first year of life, before it becomes sexually mature. However, in some populations there is a delay in the onset of metamorphosis for one or more years, although the timing of the onset of sexual maturity does not vary. As a result, they retain some juvenile features as adults, but eventually they do metamorphose. The identification of paedomorphic fossil salamanders in rocks more than 300 million years old indicates that this process has been going on for a very long time.
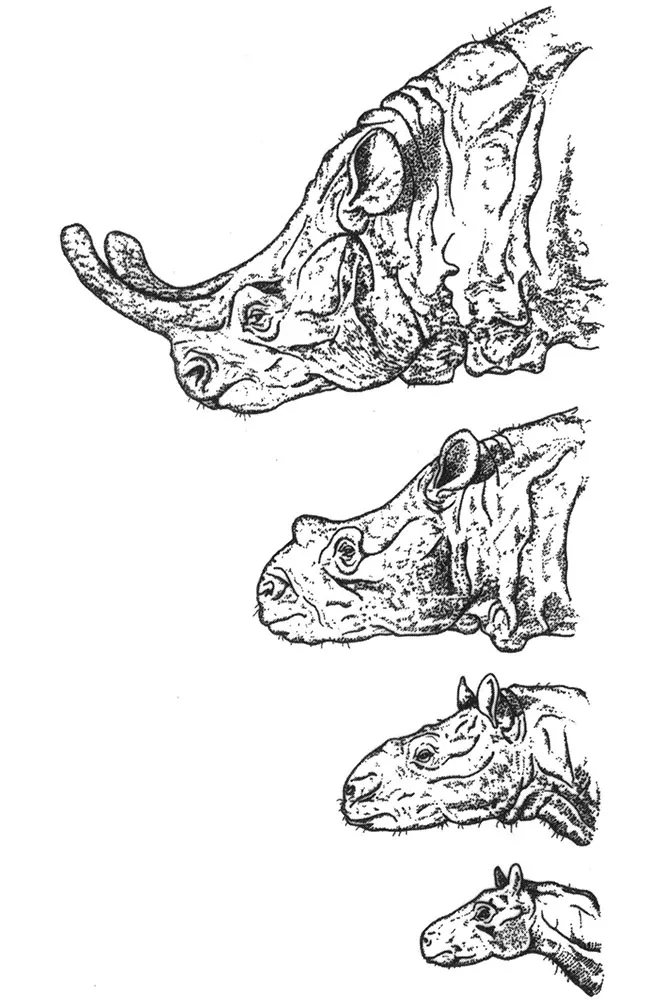
Such changes to the time of transition from one growth stage to another are an important aspect of evolution. For instance, a modern reinterpretation of yet another evolutionary ‘classic’, the extinct North American brontotheres, can be explained in much the same way, but, rather than becoming more juvenile, successive species developed ‘beyond’ their ancestors—this is peramorphosis. Brontotheres are an extinct group of perissodactyl mammals that lived 30 to 50 million years ago. They are characterised by the attainment of huge body sizes for the time, and the presence in later, larger species of bony horns that projected from the front of the skull. They were the subject of a classic monographic study published by Henry Osborn in 1929.
The earliest brontotheres that lived during the early Eocene, some 50 million years ago, were small and lacked horns. Later species were larger, and evolved horns that were disproportionately longer, relative to increasing skull size. The largest of the brontotheres, species of Brontotherium that lived during the Oligocene Period, sported huge horns. These evolutionary trends in body and horn size became one of the classic examples of a vertebrate macroevolutionary trend, in particular one that evoked ‘Cope’s Rule’, i.e. trend towards progressive increase in size. The evolution of large body and horn size was seen as being adaptive selection for massive size and structures that would have been used in head-to-head ramming during intraspecific combat.
The disproportionate changes in shape of the horns have long been cited as the classic example of allometric scaling, which means that a structure becomes larger or smaller relative to some other structure. In this case, the horn became larger, relative to the skull. However, in 1985, Mike McKinney of the University of Tennessee and Robert Schoch of Yale University published a study that they had undertaken of the role of heterochrony in the evolution of the brontothere horns. Using the detailed dimensions that Osborn had published, they argued that the situation was far more complicated than just a simple case of allometric scaling. They calculated that for the horns to increase in size to the extent that they did, there must have been not only an extension of the period of growth, but also an acceleration in the growth rate. Moreover, horn growth was initiated progressively earlier, in successive species. This resulted in horns starting to grow earlier, faster and for a longer period in successively younger species. This, argued McKinney and Schoch, was the explanation for the brontotheres’ enormous size.
Eleven years after McKinney and Schoch’s paper was published, Osborn’s data came into its own again. This time the person interpreting the data was Gerald Bales of Western University of Health Sciences in Pomona, California. Bales argued that an aspect of the way these horns may have developed that had not been considered in the past was the effect of changes to the life history stages, in particular changes to their duration. He suggested that by comparison with a wide range of large modern mammals, it was possible to estimate the times of gestation, and thus durations, of the embryonic and juvenile periods of growth of these extinct brontotheres. It is well known that a number of basic generalisations can be made concerning life history stages in living mammals. For instance, the adult size reflects the spacing of developmental events, including birth and onset of sexual maturity. Moreover, developmental phases occupy a constant proportion of the animals’ life span. Thus larger species that live longer have longer gestation periods and take longer to attain sexual maturity than smaller forms. Another well-established constant is that the duration of embryonic development occupies about 2 per cent of a mammals lifespan, while sexual maturity is attained after approximately one-tenth of the animal’s life span has been passed through. Bales therefore argued on the basis of these values that the different-sized brontotheres had life history stages of different durations. He further suggested that horn growth may have accelerated late in development. Consequently, by extending the growth period, this late acceleration could have proceeded for a longer period, producing larger horns.
One of the first groups of fossils to be used in...