
- 516 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Heterogeneous Catalysis for Energy Applications
About this book
Heterogeneous catalysis plays a central role in the global energy paradigm, with practically all energy-related process relying on a catalyst at a certain point. The application of heterogeneous catalysts will be of paramount importance to achieve the transition towards low carbon and sustainable societies. This book provides an overview of the design, limitations and challenges of heterogeneous catalysts for energy applications. In an attempt to cover a broad spectrum of scenarios, the book considers traditional processes linked to fossil fuels such as reforming and hydrocracking, as well as catalysis for sustainable energy applications such as hydrogen production, photocatalysis, biomass upgrading and conversion of CO2 to clean fuels. Novel approaches in catalysts design are covered, including microchannel reactors and structured catalysts, catalytic membranes and ionic liquids. With contributions from leaders in the field, Heterogeneous Catalysis for Energy Applications will be an essential toolkit for chemists, physicists, chemical engineers and industrials working on energy.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
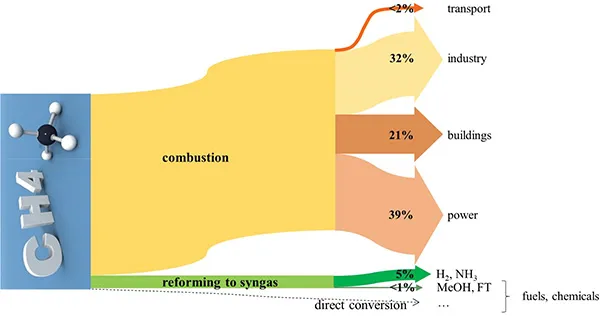
Natural gas consumption | 1990 | 2017 | 2040 |
Transport | 2 | 55 | 192 |
Industry | 771 | 1182 | 1749 |
Buildings | 530 | 784 | 971 |
Power | 543 | 1443 | 2109 |
Non-combusted | 103 | 206 | 349 |
Total | 1949 | 3670 | 5370 |
CH4 + H2O → 3H2 + CO | ∆rH°298K = 206 kJ mol–1 | (1.1) |
CH4 + CO2 → 2H2 + 2CO | ∆rH°298K = 247 kJ mol–1 | (1.2) |
CO2 + H2 → H2O + CO | ∆rH°298K = 41 kJ mol–1 | (1.3) |
CH4 + 0.5 O2 → 2H2 + CO | ∆rH°298K = –36 kJ mol–1 | (1.4) |
CH4 → C(s) + 2H2 | ∆rH°298K = 75 kJ mol–1 | (1.5) |
2CO → C(s) + CO2 | ∆rH°298K = –171 kJ mol–1 | (1.6) |
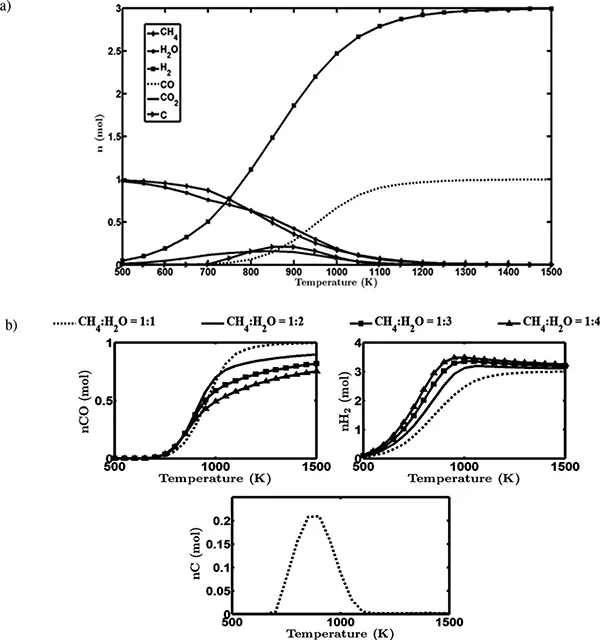
Table of contents
- Cover
- Half Title
- Series Information
- Title Page
- Copyright Page
- Preface
- Contents
- Chapter 1 Design of Advanced Catalysts for Natural Gas Reforming Reactions
- Chapter 2 Natural Clay Minerals for Hydrocracking Reactions
- Chapter 3 Catalytic Conversion of Fossil and Renewable Fuel Resources: Approaches Using Sub and Supercritical Water as a Reaction Medium
- Chapter 4 Recent Advances in Photocatalytic Materials for Solar Fuel Production from Water and Carbon Dioxide
- Chapter 5 Catalytic Technologies for Clean Hydrogen Production
- Chapter 6 Application of Heterogeneous Catalysts for the Conversion of Biomass-derived Feedstocks into Fuel Components and Eco-additives
- Chapter 7 Catalysis in Modern Bio-refineries: Towards a New Bio-energy Paradigm
- Chapter 8 Catalytic Technologies for the Production of Liquid Transportation Fuels from Biomass
- Chapter 9 Metal Organic Frameworks: From Material Chemistry to Catalytic Applications
- Chapter 10 Application of Ionic Liquids for Sustainable Catalysis
- Chapter 11 Structured Catalysts and Non-conventional Reactor Designs for Energy Applications
- Chapter 12 Catalytic Conversion of CO2 to Fuels and Value-added Chemicals
- Chapter 13 In Situ Characterization of Metal/Oxide Catalysts for CO2 Conversion: From Fundamental Aspects to Real Catalyst Design
- Chapter 14 Catalytic Aspects of Fuel Cells: Overview and Insights into Solid Oxide Fuel Cells
- Subject Index