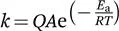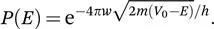CLÁUDIO M. NUNES,*a IGOR REVA*a AND RUI FAUSTO*a,b
1.1 Introduction
The theoretical foundations for nuclei and electron tunnelling were put forward by Hund,1 Wigner,2 Bell,3 and others,4,5 following the establishment of quantum mechanics. A more generalized treatment of tunnelling in chemistry appeared almost half a century afterwards in the seminal Bell's monography “The Tunnel Effect in Chemistry”.6,7 Indeed, as addressed in several other chapters of this book, theoretical methodologies to treat quantum mechanical tunnelling (QMT) in chemical reactions are still being developed nowadays. In this chapter, QMT in chemistry will be addressed from a more experimental perspective, taking advantage of the conditions typical of a matrix isolation experiment, which allow for direct observation of tunnelling driven processes by steady-state spectroscopic methods.
A simple and common way to portray tunnelling, although not particularly accurate,8 is to consider it as a phenomenon that arises from the wave–particle duality. If in a chemical reaction the moving distance of a nucleus is comparable to its de Broglie wavelength, then there is a non-negligible probability of finding the nucleus on the other side of the reaction barrier, even if the system does not possess enough thermal energy to surmount the barrier. It means that nuclei are able to penetrate through reaction barriers. Of course, such unexpected behavior is framed on a classic perspective, in which all atoms involved in a chemical transformation are assumed to behave as hard spheres.
According to the classic transition state theory (TST), reactants must acquire enough energy to overcome a barrier in order to give rise to products.9–11 Statistically, as temperature increases, more molecules will have enough energy to traverse the barrier, so that the reaction rate typically increases proportionally. Such temperature dependence of reaction rates was empirically established by Arrhenius in his well-known equation [eqn (1.1)], long before the development of the TST.11,12
In eqn (1.1), A is a pre-exponential constant, Ea the activation energy (J mol−1), R the universal gas constant (8.314 J mol−1 K−1), and T (K) is the absolute temperature.
However, deviations from the Arrhenius typical behavior can take place if tunnelling occurs simultaneously with the classic passage over the barrier. In these cases, the QMT contribution to the reaction rate can be incorporated using a tunnelling correction factor Q in the kinetic models, as it is, for instance, shown in eqn (1.2).6,11,13
The tunnelling correction factor Q takes into account the tunnelling permeability through the barrier, which depends on the mass of the tunnelling particle, as well as on the barrier height and width.6,13
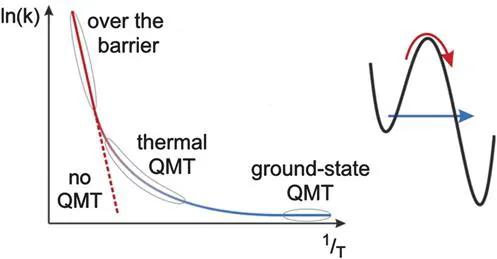
The existence of QMT contribution to a chemical reaction is typically detected indirectly by the observation of non-linear Arrhenius plots or abnormal kinetic isotope effects.14–18 The temperature dependence of k in eqn (1.1) is given by the exponential factor, exp(−Ea/RT). Consequently, a plot of ln(k) against 1/T results in a straight line (see Figure 1.1). Its slope is −Ea/R. For historic reasons, such plots are referred to as Arrhenius plots. On the other hand, contrary to the classical over-the-barrier thermal process, tunnelling rates are approximately independent of the temperature. For a low enough temperature, when the system is in its ground vibrational state, the overall reaction rate is dominated by tunnelling and, consequently, temperature independent (see Figure 1.1).
Figure 1.1 Logarithm of the rate constant plotted versus the inverse temperature (Arrhenius plot). The classical (thermal) over-the-barrier reaction results in a straight line. The rate becomes constant at low temperature when ground-state quantum-mechanical tunnelling (QMT) dominates. Adapted from ref. 15 with permission from the Royal Society of Chemistry.
Working at low temperatures is in fact a very convenient way to search for evidence of tunnelling in chemical reactions. At cryogenic temperatures (e.g., 3–10 K), thermally activated rates become negligible for systems having barriers as low as ∼4 kJ mol−1 (∼1 kcal mol−1), so the occurrence of a chemical transformation can only be due to a “pure” tunnelling reaction.14–18 If such tunnelling transformations span from seconds to days, they can be directly observed and monitored using stationary-state spectroscopy methods. Indeed, particularly during the last decade, direct spectroscopic evidence of a variety of tunnelling-driven reactions has been reported using the low-temperature matrix isolation technique coupled to infrared spectroscopy. These observations have contributed significantly to a better understanding of QMT and its role in chemistry.19
In this chapter, we will address some representative cases of tunnelling-driven chemical processes, from conformational isomerizations to H-atom and heavy-atom bond-breaking/bond-forming reactions occurring in organic molecules under matrix isolation conditions. Examples of tunnelling reactions at cryogenic temperatures taking place in other than matrix isolation conditions are outside the scope of this chapter.
1.2 Description of Simple Mathematic Models for Tunnelling Computations
The present chapter is not concerned with the theory of tunnelling. There are several recent reviews on the topic.18,20–22 Here, we shall recall that any occurrence of a tunnelling reaction must always face a barrier to overcome. This section will present simple formulas for the probabilities of tunnelling through two barriers of different shapes.
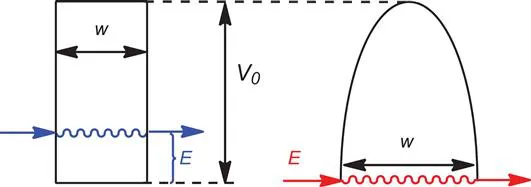
In a recent review,22 Borden presents the formula for the energy-dependent probability P(E), of a particle with mass m, tunnelling through a rectangular barrier of width w that is V0−E higher than the energy of the particle (see Figure 1.2, left):
Figure 1.2 Left: tunnelling through a rectangular barrier of width w, at an energy V0−E below the top of the barrier. Right: tunnelling through a parabolic barrier of width w, at an energy V0−E below the top of the barrier.
A more realistic barrier shape is that of the inverted parabola (as in Figure 1.2, right). The approximate solutions for the equations describing the tunnelling of a particle through a parabolic barrier were independently devised by Wentzel, Kramers, and Brillouin in 1926.23–25 As it is noted by Borden,22 “what has become known as the WKB approximate solution23–25to the calculation of the probability of tunnelling through a parabolic barrier should really be known as the JWKB approximate solution”,22 because “earlier Jeffreys26had published the mathematics necessary to obtain approximate solutions to differ...