
Introduction to Stereochemistry
- 182 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Introduction to Stereochemistry
About this book
CHEMISTRY STUDENT GUIDES. GUIDED BY STUDENTS
Why did the drug thalidomide cause birth defects? What is the chemical difference between sucrose and lactose in your food? Stereochemistry holds the answer and is essential to the understanding of the chemistry of life.
Stereochemistry is an important concept that often causes confusion amongst students when they learn it for the first time. Unlike most other areas of chemistry, it requires the chemist to visualise molecules in 3D, which can be difficult. In this book we deal with tricky concepts like conformation and configuration, how to represent them accurately and how to use the correct terms to describe them in both organic and inorganic chemistry. We involved students in the writing process to ensure we deal with areas that you find difficult, in an understandable language. With problems designed to focus on common errors and misconceptions, real life examples, and practical hands-on exercises coupled with visualisation tips, our intention is to give you the tools to become confident in stererochemistry.
Complementing mainstream organic textbooks, or self-study, this book is for anyone who has struggled with describing alkenes as E or Z, assigning R and S absolute configurations, drawing Newman projections or chair representations of cyclohexanes, axial chirality, understanding the stereochemistry of octahedral metal complexes and indeed explaining complexities observed in NMR spectra.
Chemistry Student Guides are written with current students involved at every stage, guiding the books towards the most challenging aspects of the topic. Student co-authors for Introduction to Stereochemistry are Caroline Akamune, Michael Lloyd and Matthew Taylor.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
CHAPTER

What You Will Get from This Chapter
Stereochemistry
1.1 Thalidomide – Why Stereochemistry Is Important
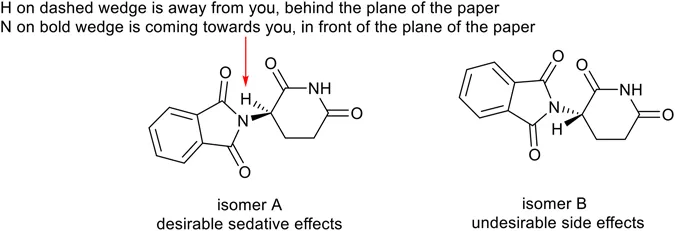
1.2 Stereoisomerism
1.2.1 Configurational Isomerism
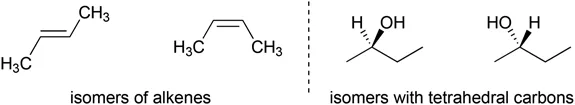
1.2.2 Conformational Isomerism
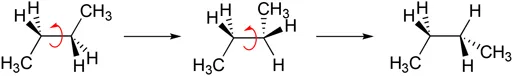

1.3 Representing Molecules in 3D
1.3.1 New Ways of Drawing Molecules
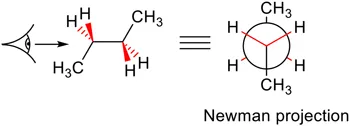
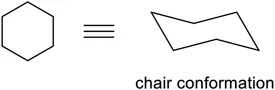
1.3.2 Stere...
Table of contents
- Cover
- Title
- Copyright
- Author Biographies
- Preface
- How to Use This Book
- Glossary
- Table of Contents
- 1 Stereochemistry
- 2 Alkenes
- 3 Stereogenic Centres, Enantiomers and Diastereoisomers
- 4 Conformation of Acyclic Compounds
- 5 Conformation of Cyclic Compounds
- 6 Chiral Molecules Without a Stereogenic Atom
- 7 Stereochemistry of Inorganic Molecules
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app