
- 408 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
About this book
Bone Marrow Processing and Purging: A Practical Guide provides an up-to-date practical guide to the major ex vivo procedures associated with bone marrow transplantation. Previously, this information was communicated primarily by word of mouth; now experts in the field present detailed descriptions and evaluations of methods for marrow harvesting, evaluation (including tumor infiltration, flow cytometric analysis, and colony assays), comparative methods for automated nucleated cell separation and enumeration, tumor cell purging, T cell depletion, stem cell selection, gene transfer, and cytopreservation. Special sections address quality control and FDA regulations.
The book provides a unique information source intended for clinicians, researchers, technical staff, transplant nurses, and medical students involved in this rapidly expanding area of medicine.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Part 1: Introduction and General Guidelines
Chapter 1
AN INTRODUCTION TO BONE MARROW TRANSPLANTATION AND PROCESSING
A. John Barrett
TABLE OF CONTENTS
I. History of Clinical Bone Marrow Transplantation
II. Allogeneic BMT
III. Autologous BMT
IV. Current Indications and Use of Bone Marrow Transplantation
V. BMT as a Cure for Malignant Disease
VI. BMT as a Cure for Nonmalignant Disorders
VII. Requirements for Hemopoietic and Immune Reconstitution
VIII. Purging of Tumor Cells from Bone Marrow
IX. GvHD and T Cell Depletion
X. Conclusion — the Place of Purging in BMT
Acknowledgment
References
I. HISTORY OF CLINICAL BONE MARROW TRANSPLANTATION
The first attempts at bone marrow transplantation (BMT) in man followed rapidly upon the realization and subsequent proof by Lorenz et al. that the radiation-protective effect conferred by bone marrow in mice was due to engraftment and proliferation of viable bone marrow cells.1 At a very early stage, two distinct approaches emerged: autologous BMT, used in patients with malignant disease as a means of intensifying anticancer treatment, and allogeneic BMT, first employed in the treatment of bone marrow failure, and in patients with uncontrolled leukemia.
II. ALLOGENEIC BMT
In the 1950s, attempts to use bone marrow from random donors to rescue patients with aplastic anemia or bone marrow failure resulting from radiation accidents were carried out. The only successes occurred in three out of seven patients with severe aplastic anemia receiving bone marrow from an identical twin.2
Use of random donors as a source of bone marrow led most frequently to death from failure of engraftment, or from so-called “secondary disease” (now recognized to be graft-vs.-host disease — GvHD). However, Mathé reported transient engraftment from multiple donor marrow infusions in six radiation workers exposed to varying amounts of ionizing radiation.3 It was not until the advent of tissue typing and compatibility testing that substantial numbers of patients benefited from BMT procedures. The decade of the 1970s was characterized by clinical trials which identified the place of BMT in the treatment of a wide number of malignant and nonmalignant disorders. Standardized approaches to pretransplant preparative regimens and prevention of GvHD were developed, and, by 1980, allogeneic BMT from matched sibling donors would be expected to achieve disease-free survivals in hematological malignancies in favorable circumstances in over 50% of patients. The last decade has seen the application of biotechnology to clinical BMT, notably, attempts to prevent and treat GvHD with monoclonal antibodies, and the increased use of hemopoietic growth factors and recombinant cytokines to promote hemopoietic and immune reconstitution. In this context, the prevention of GvHD by in vitro depletion of T lymphocytes has played a prominent role.4 The challenges confronting allogeneic BMT are a continued high procedural mortality rate due to the conditioning regimen and GvHD and the inability to safely transplant marrow across major histocompatibility barriers, which largely restrict its use to patients with HLA-matched siblings. Available technology is not yet able to allow either selection of immune recovery to favor an immunological response to minimal residual disease (graft-vs.-leukemia effect) or restoration of immune competence against microorganisms.
III. AUTOLOGOUS BMT
The use of autologous bone marrow as a source of regenerative hemopoietic cells was first reported in 1958 by Kurnick et al.5 At this time, the concepts of tumor kill, derived from studies of radiation survival of malignant cells in mice, indicated that increased intensity of treatment would permit the log kill of a tumor mass beyond the last viable cell.6 Intensive high-dose chemotherapy followed by autologous BMT (ABMT) appeared to offer the chance of curing malignancies in situations where marrow failure prevented the use of sufficient chemotherapy without marrow rescue. There were several reasons for the lack of success of these early studies. First, the use of high-dose chemo- or radiotherapy was in its infancy, and many of the treatments used would be judged inadequate by standards of today. Second, the type of treatment used was limited by the need to retransfuse viable noncryopreserved marrow cells within 48 h of collection, thus permitting only short, single agent therapies to treat malignancies.
There was resumed interest in autologous BMT in the last decade, concurrent with increased confidence in the technique of allogeneic BMT and development of reliable techniques for bone marrow cryopreservation and storage. In Europe, the main area of application for ABMT has been the leukemias and lymphomas, while in North America, it is most frequently applied in the treatment of pediatric and adult solid tumors.7 The major challenges still confronting the technique of ABMT are the limitation of available high-intensity treatments to eradicate disease without causing irreversible damage to nonhemopoietic organs, and the possibility that disease relapse following ABMT is related either to the reinfusion of a small number of malignant cells in the marrow innoculum or the lack of an immunological “graft-vs.-leukemia” effect.
IV. CURRENT INDICATIONS AND USE OF BONE MARROW TRANSPLANTATION
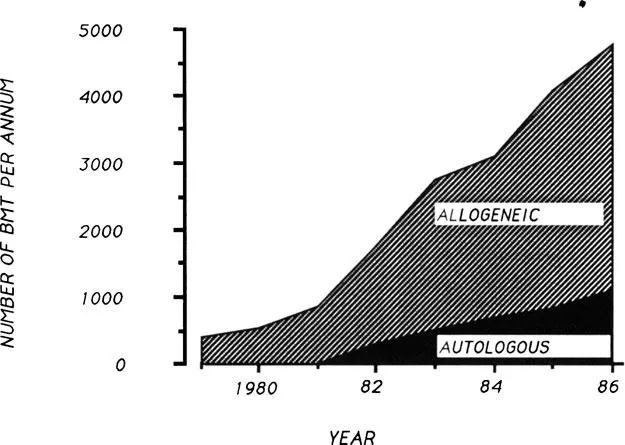
BMT has a place in the treatment of a wide number of malignant and nonmalignant disorders, including both congenital and acquired diseases (Table 1). The International Bone Marrow Transplant Registry (IBMTR) receives information from a large proportion of active BMT teams worldwide and serves as a useful source of BMT statistics. Figure 1 shows the indications for BMT represented proportionally. The leukemias represent the most frequent indication for allogeneic BMT, with all other indications accounting for the remaining 25% of BMT activity. Autologous BMT and allogeneic BMT reporting rates are increasing steadily (Figure 2) due to both an ever-increasing number of BMT teams worldwide and to an increased number of transplants per team.4 It has been estimated that the IBMTR receives notification of approximately 50% of all BMT activity. Their figures suggest that over 10,000 BMTs are currently carried out per annum, even at a conservative estimate.8
Malignant disorders | Nonmalignant disorders |
|---|---|
Leukemias 1,2 | Bone marrow failure syndromes 1 |
Acute myeloblastic leukemia | Acquired severe aplastic anemia |
Acute lymphoblastic leukemia | Fanconi aplasia |
Chronic myelogenous leukemia | Reticular dysgenesis |
Myelodysplastic syndromes | Immunodeficiency states 1 |
Acute myelofibrosis | Severe combined immunodeficiency |
Lymphoproliferative disorders | Wiskott Aldrich syndrome |
Non-Hodgkins lymphoma 2 | Some less severe combined ID disorders |
Hodgkins lymphoma 2 | Hematological disorders 1 |
Multiple myeloma 1,2 | Thalassemia syndromes |
Chronic lymphocytic leukemia 2 | Some sickle cell anemias |
Solid tumors 2 | Congenital neutropenia |
Neuroblastoma | Severe congenital platelet disorders |
Bronchial carcinoma | Osteopetrosis |
Breast carcinoma | Gaucher disease |
Melanoma | Nonhematological genetic disorders 1 |
Cerebral tumors | Mucopolysaccharidoses |
Osteosarcoma | Leucodystrophies |
Ewings sarcoma | Other rare metabolic disorders |
Teratomas | |
Others |
Type of transplant used: 1, Allografts, 2, autograft.

V. BMT AS A CURE FOR MALIGNANT DISEASE
Cure is achieved by high-dose chemotherapy or radiotherapy treatments used to reduce tumor bulk to a size where either no viable cells are left or where residual disease can be contained and possibly eliminated by the immune system of the recipient. There is evidence that both these processes are important.9
Numerous animal experiments demonstrate that the possibility of eliminating an experimentally induced tumor relates directly to the total dose of radiation administered. A...
Table of contents
- Cover
- Title Page
- Copyright Page
- Preface
- The Editor
- Contributors
- Table of Contents
- Part 1: Introduction and General Guidelines
- Part 2: Harvesting, Shipping, and Nucleated Cell Separation
- Part 3: Bone Marrow Evaluation
- Part 4: Lymphocyte Depletion for Allogeneic Transplantation
- Part 5: Autologous Bone Marrow Purging
- Part 6: Marrow Cryopreservation and Future Trends
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Bone Marrow Processing and Purging by Adrian P. Gee in PDF and/or ePUB format, as well as other popular books in Medicine & Hematology. We have over one million books available in our catalogue for you to explore.