
- 278 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
Biopharmaceuticals: Challenges and Opportunities
This book highlights how the traditional microbial process technology has been upgraded for the production of biologic drugs how manufacturing processes have evolved to meet the global market demand with quality products under the guidelines of internally recognized regulatory bodies.
It also carries information on how, armed with a deeper understanding of life-threatening diseases, biopharmaceutical companies and the life sciences industry have developed formal and informal partnerships with researchers in institutes, universities, and other R&D organizations to fulfil timely, quality production with perfect safety and security. One of the most interesting aspects of this book is the conceptual development of personalized medicine (or precision medicine) to provide the right treatment to the right patient, at the right dose at an earlier stage of development, for genetic diseases. Besides this, it also highlights the most challenging aspects of modern biopharmaceutical science, focusing on the hot topics such as design and development of biologic drugs; the use of diversified groups of host cells belonging to animals, plants, microbes, insects, and mammals; stem cell therapy and gene therapy; supply chain management of biopharmaceuticals; and the future scope of biopharmaceutical industry development.
This book is the latest resource for a wide circle of scientists, students, and researchers involved in understanding and implementing the knowledge of biopharmaceuticals to develop life-saving biologic drugs and to bring awareness to the development of personalized treatment that can potentially offer patients a faster diagnosis, fewer side effects, and better outcomes.
Features:
- Explains how the traditional cell culture methodology has been changed to a fully continuous or partially continuous process
- Explains how to design and fabricate living organs of body by 3D bioprinting technology
- Focuses on how a biopharmaceutical company deals with various problems of regulatory bodies and develops innovative biologic drugs
- Narrates in detail the updated information on stem cell therapy and gene therapy
- Explains the development strategies and clinical significance of biosimilars and biobetters
- Highlights the supply chain management of biopharmaceuticals
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1 Biopharmaceuticals – A New Frontier
1.1 Continuous Manufacturing
1.1.1 Fully Integrated Continuous Process
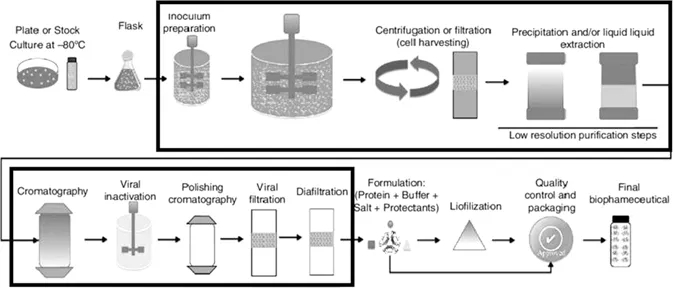
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Author
- Chapter 1 Biopharmaceuticals – A New Frontier
- Chapter 2 Biopharmaceutical Production
- Chapter 3 Transition from Pharmaceutical Plants to Biopharma Sector
- Chapter 4 Biopharmaceuticals from Animal Cells
- Chapter 5 Plant-Derived Therapeutic Biomolecules
- Chapter 6 Biosimilars
- Chapter 7 Biopharmaceutical Supply Chain Management
- Index