
- English
- ePUB (mobile friendly)
- Available on iOS & Android
eBook - ePub
Crustacean Egg Production
About this book
This title discusses egg formation, release, and development, variations in life history patterns, population, and fisheries aspects regarding crustaceans.
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
Topic
Sciences biologiquesSubtopic
Biologie1. Egg formation, release and development
Egg production, release and activation in the marine shrimp, Sicyonia ingentis
Wallis H. Clark, Jr. & Muralidharan C. Pillai
Department of Animal Science & Bodega Marine Laboratory, University of California, Bodega Bay, USA
ABSTRACT
The decapod Sicyonia ingentis reproduces from the middle of June to the middle of October, during which the females undergo ovarian development and spawning. Multiple spawning without an intervening molt commonly occurs among both field and laboratory held populations. While the details on the vitellogenic stages of oögenesis are largely unknown, post-vitellogenic stages of oöcyte development have been characterized. During the late stages of oögenesis, large jelly-like inclusions (jelly precursor) develop in the oöplasm and eventually become externalized in deep extra-oöcytic crypts around the periphery of the oöcyte. This stage of the oögenesis (cortical specialization phase) occurs prior to germinal vesicle break down (GVBD). GVBD occurs before ovulation and spawning and is an asynchronous event. Prior to spawning, the females exhibit a characteristic prespawning behavior which is correlated with ovulation. The ovulated females can be induced to spawn on demand under laboratory conditions; upon contact with sea water, spawned ova undergo a series of activational events that include: 1) release of the jelly precursor from the extra-oöcytic crypts, 2) formation of a jelly layer, 3) resumption of meiosis, and 4) formation of an extracellular hatching envelope. These events are Mg++ dependent in fertilized ova; however, in unfertilized ova both Mg++ and Ca++ are required.
1 INTRODUCTION
The order Decapoda is divided into two suborders, the Pleocyemata, animals that brood their eggs, and the Dendrobranchiata, whose members are broadcast spawners. Although much is known concerning the reproductive biology of the Pleocyemata, a basic understanding of the reproductive biology of the Dendrobranchiata is still needed. This is somewhat curious, since members of this suborder, especially the Penaeoidea, represent one of the largest wild caught and cultured fisheries in the world. With the exception of older treatises by Hudinaga (1942) and King (1948), most of the available literature deals with techniques for the induction of ovarian growth (i.e. eyestalk ablation as well as nutritional and environmental requirements). While these studies have been important to culturists, for the most part, they have done little towards furthering our understanding of the basic process associated with egg production and early development.
The most thoroughly examined Penaeoidea is the marine shrimp, Sicyonia ingentis (Clark et al. 1984). Extensive knowledge is available on the reproductive biology of males, including studies on male gamete formation (Shigekawa & Clark 1986), sperm structure and sperm activation (for review see: Clark & Griffin 1988). Although less is known concerning the reproductive biology of the female, the reproductive cycle, postvitellogenic ovarian development (Anderson et al. 1985) and egg activation (Pillai & Clark 1987) have been studied. This paper reviews our knowledge of female reproductive biology in this species.
2 BIOGEOGRAPHY
Sicyonia ingentis is the only member of its genus recorded from the west coast of the United States. Its geographic range extends from Monterey Bay, California in the north to Isla Maria Madre, Mexico in the south; in addition it is also reported to be found in the Gulf of California (Perez Farfante 1985). Sicyonia ingentis is commonly found at depths of 61 to 183 m (Carlisle 1969). Females of this species attain a maximum length of 180 mm, measured from the telson to the base of the antenna. Males, on the other hand, are generally smaller and attain a maximum length of 157 mm (Herkelrath 1977). This species is commercially fished in the Santa Barbara Channel off California.
3 FIELD BIOLOGY
The reproductive season of S. ingentis females, sampled off Santa Barbara, California, between 1979-1981, extended from the middle of June to the middle of October. During this period the female population entered anecdysis; ecdysis resumed at the end of the reproductive season. Females exhibited two periods of molt activity, one between late February and early May and a second, more extended period, between late October and early November (Fig. 1). Males did not show the dramatic peaks in molt activity demonstrated by the females, though they did undergo anecdysis during the summer months (Anderson et al. 1985).
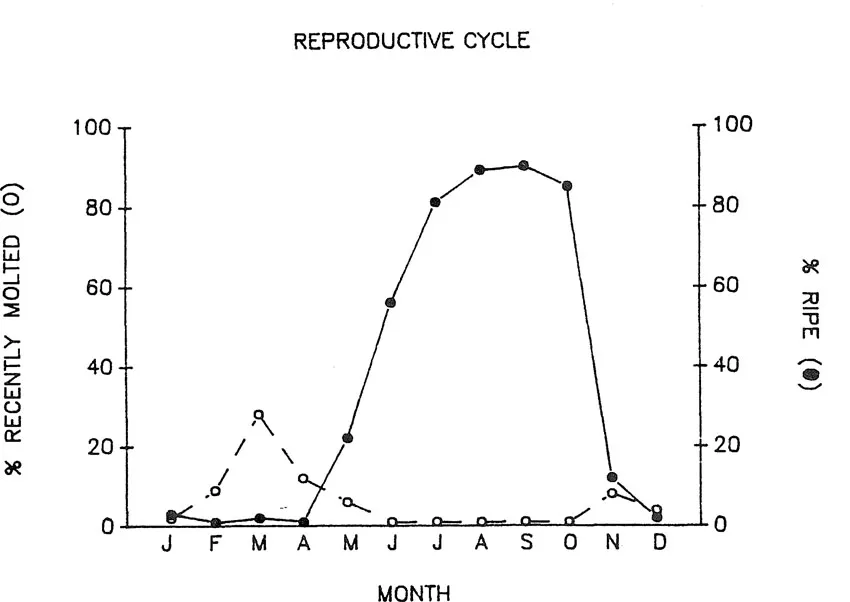
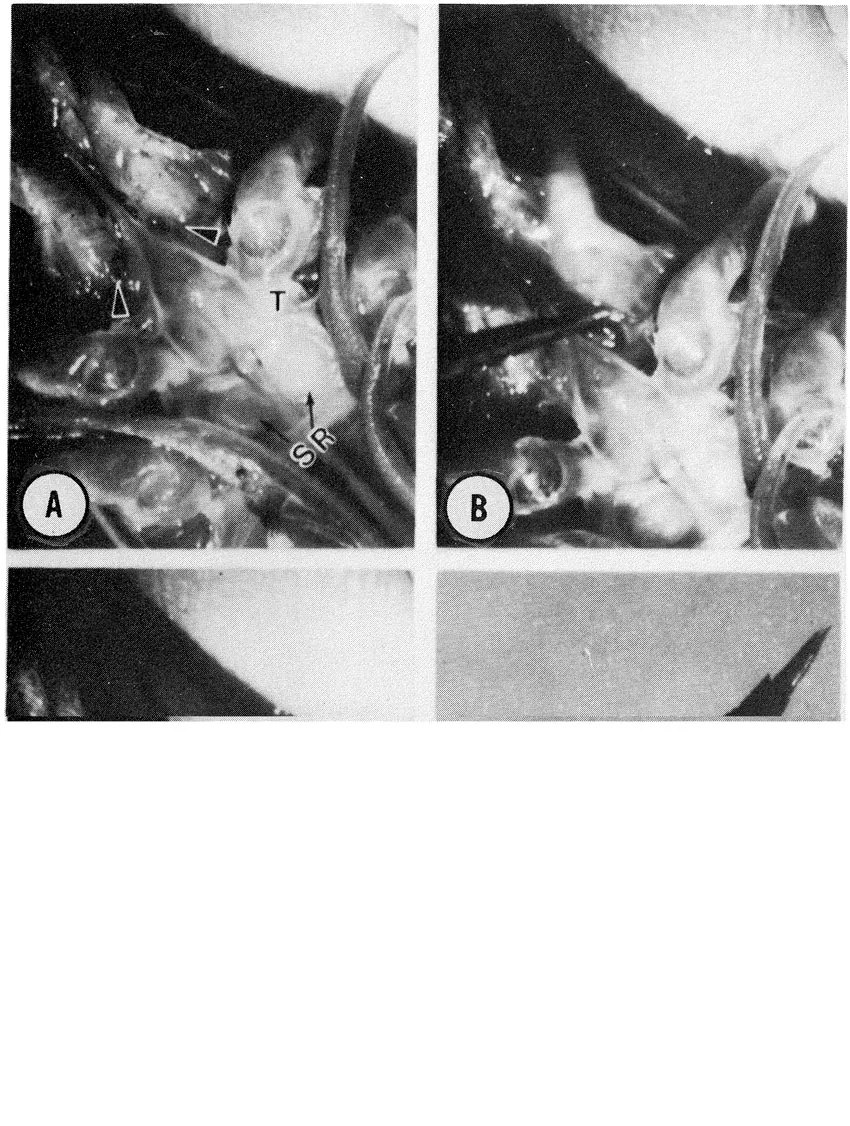
Anderson et al. (1985) suggested that females undergo multiple spawns within a single molt cycle. This was based on the high frequency of females in wild population in advanced stages of oöcytic development throughout the reproductive season. This hypothesis was supported by multiple spawning, without intervening molts of laboratory held S. ingentis. The average time between spawns for a single female was 20 days. Multiple spawns without an intervening molt have also been reported for laboratory held Penaeus indicus (Emmerson 1980).
4 EGG DEVELOPMENT
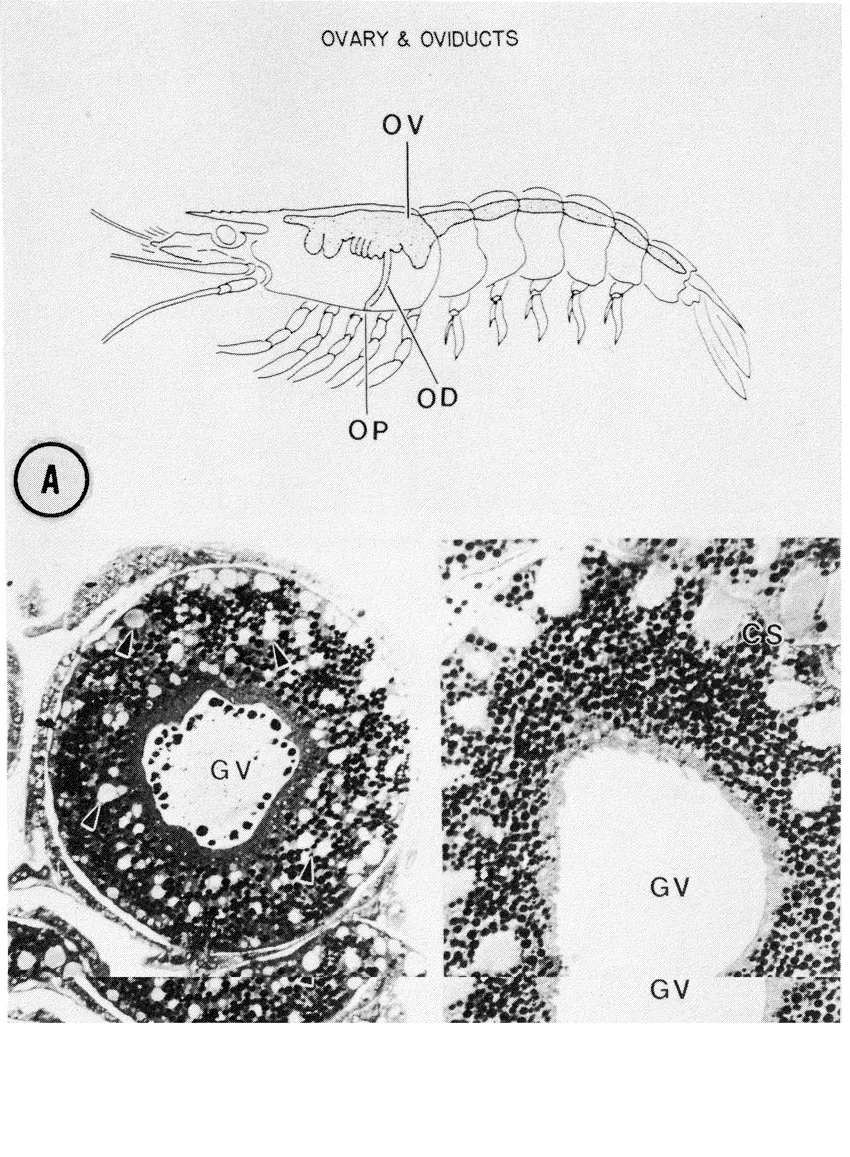
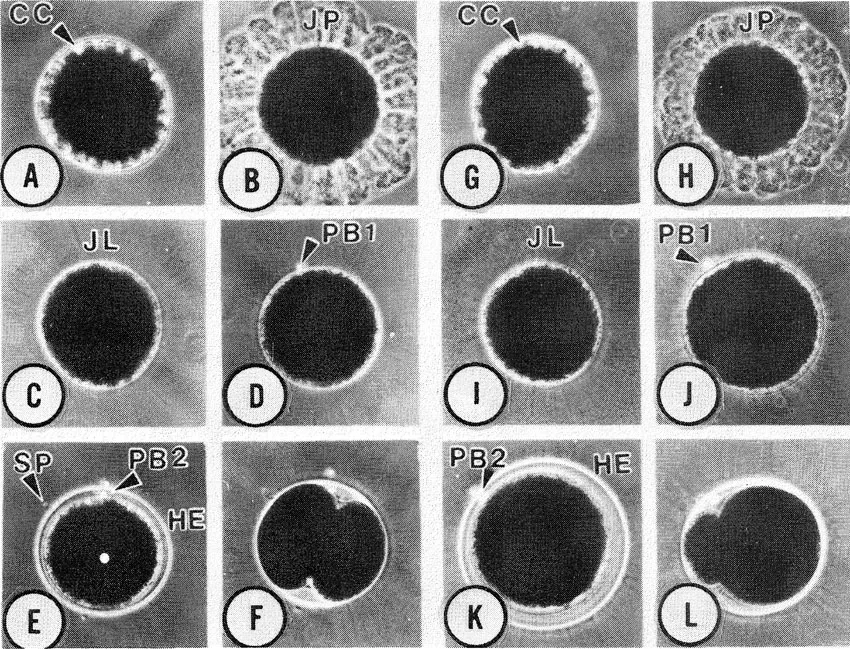
The highly fecund S. ingentis possesses bilateral ovaries which extend from the anterior region of the cephalothorax to the telson. Lateral extensions of the anterior lobes in the cephalothorax course in a ventrolateral direction. Oviducts pass from each anterior lobe to an ovipore situated at the base of each third pereiopod (Pl. 1A). With the exception of the gross morphology, however, the structural organization of the ovary is largely unknown. The vitellogenic stages of oogenesis also remain an enigma. For example, it is not known whether yolk production is autosynthetic, sequestered from extra oöcytic sources (e.g. pinocytotic uptake from hemolymph), or if both mechanisms occur. In the oöcytes of Penaeus sp. both mechanisms may in fact be used (Duronslet et al. 1975). The vitellogenic stages in the oöcytes of S. ingentis appear similar to the vitellogenic stages of Penaeus sp.; however, preliminary data for S. ingentis suggests that, what has been described as autosynthetic yolk production in Penaeus sp., may be the production of a jelly precursor (unpubl. data).
During the late stages of oögenesis, large inclusions appear within the oöplasm (Pl. 1B); these structures fuse with each other, forming rod-shaped bodies which become externalized in deep oöplasmic crypts around the periphery of the oöcytes (Pl. 1C).
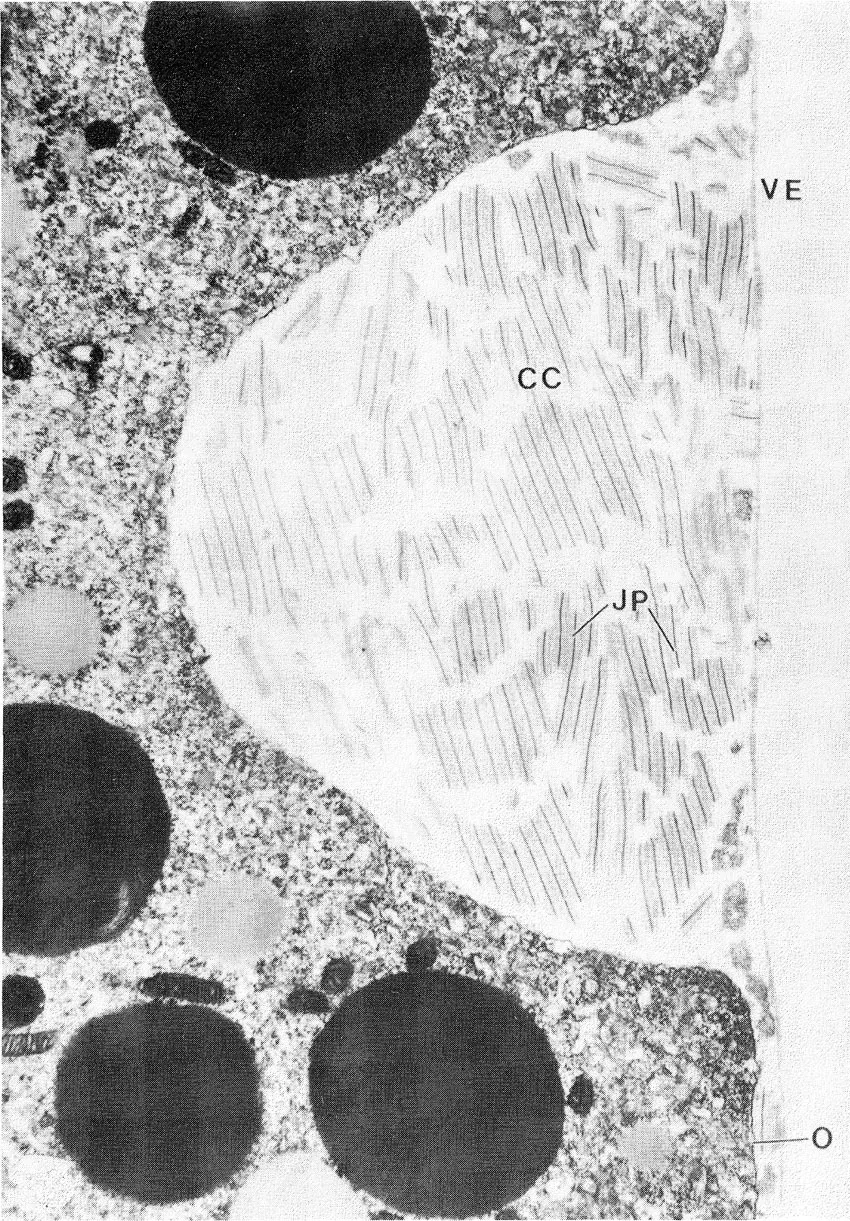
The rod-shaped bodies in S. ingentis possess feather-like substructural elements (Pl. 3) similar to that described in Penaeus sp. (Duronslet et al. 1975, Clark et al. 1980, Lynn & Clark 1987). These elements appear to be of oöcytic origin; this is interesting in view of recent evidence suggesting that similar substructural material, of follicular origin, is responsible for extracellular coats of lobster ova (Talbot & Goudeau 1988).
The appearance of jelly-like inclusions around the cortex of penaeoidean oöcytes [corticle specialization phase (CS)] apparently represents one of the last postvitellogenic ...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Table of Contents
- Preface
- Introduction
- 1 Egg formation, release and development
- 2 Variations in life history patterns
- 3 Population and fisheries aspects
- List of authors
- Index
Frequently asked questions
Yes, you can cancel anytime from the Subscription tab in your account settings on the Perlego website. Your subscription will stay active until the end of your current billing period. Learn how to cancel your subscription
No, books cannot be downloaded as external files, such as PDFs, for use outside of Perlego. However, you can download books within the Perlego app for offline reading on mobile or tablet. Learn how to download books offline
Perlego offers two plans: Essential and Complete
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
We are an online textbook subscription service, where you can get access to an entire online library for less than the price of a single book per month. With over 1 million books across 990+ topics, we’ve got you covered! Learn about our mission
Look out for the read-aloud symbol on your next book to see if you can listen to it. The read-aloud tool reads text aloud for you, highlighting the text as it is being read. You can pause it, speed it up and slow it down. Learn more about Read Aloud
Yes! You can use the Perlego app on both iOS and Android devices to read anytime, anywhere — even offline. Perfect for commutes or when you’re on the go.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app
Yes, you can access Crustacean Egg Production by Armand Kuris in PDF and/or ePUB format, as well as other popular books in Sciences biologiques & Biologie. We have over one million books available in our catalogue for you to explore.