
- 576 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Immune Modulating Agents
About this book
Discussing the systemic immune response in the contexts of health, disease, and therapy, this unique resource-the only broadly based book of its kind available on the subject-offers comprehensive examinations of the pathways and agents that affect the human immune response and provides state-of-the-art presentations on practical methods of immune modulation.
Focuses on the immune response and modulation in infectious diseases, such as HIV, hepatitis, and parasitic infections and highlights immune modulating agents in gastrointestinal diseases, sepsis, cancer, and autoimmunity!
Written by over 50 international authorities representing distinguished institutions in nine countries, Immune Modulating Agents
Tools to learn more effectively

Saving Books

Keyword Search

Annotating Text

Listen to it instead
Information
1
The Immune System and Immune Modulation
I. INTRODUCTION
II. OVERALL PRINCIPLES OF IMMUNE REGULATION
A. Specific Versus Nonspecific Signals
| Degree of specificity | Definition | Examplesa |
|---|---|---|
| | ||
| Specific intervention | Modulation of monoclonal or oligoclonal response to a determined antigen | Immunization with pep-tides or proteins; application of modified antigens |
| Semispecific intervention | Intervention on a fraction of the immune repertoire determined by the expression of determined V genes | Superantigens. TCR-V/β or TCR-Vα-specific antibodies, and immunotox-ins. T cell vaccination |
| Nonspecific intervention | Application of substances that alter immune responses independently from the antigen or antigen receptor(s) involved | Application of cytokines, hormones; ablation of T cell subsets |
B. Three Existential Choices
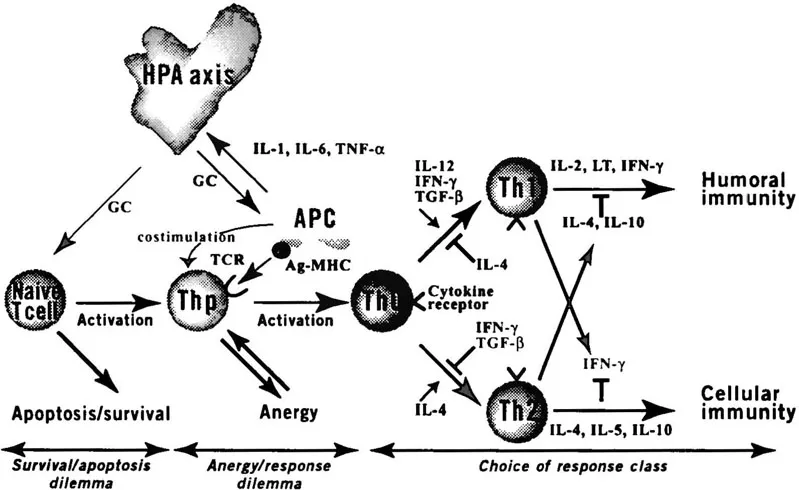
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication Page
- Preface
- Contents
- Contributors
- Part I: Immunomodulation
- Part II: Immunomodulating Agents in Disease
- Part III: Immunomodulating Agents in Therapy
- Index
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app