J. Guoa, B. H. Yuna and R. J. Tureskya
Environmental and dietary genotoxicants, endogenous electrophiles, and ionizing-radiation damage the human genome. DNA damage products include strand breaks, abasic sites, cross-links, and covalent DNA adducts. Cells have multiple enzyme systems of DNA repair to cope with many types of DNA lesions. However, DNA damage that escapes repair can induce mutations during cell division. Biomonitoring of DNA damage in humans is critical to understand the chemicals involved in mutagenesis and disease risk. DNA adducts serve as dosimeters for chemical exposure and represent a measure of the biologically effective dose. DNA-adducts are also vital biomarkers in risk assessment and aid in formulating public health policy to reduce chemical exposure and cancer incidence. This chapter describes the major technologies and the different types of biological specimens used in biomonitoring of DNA damage in humans.
1.1 Introduction
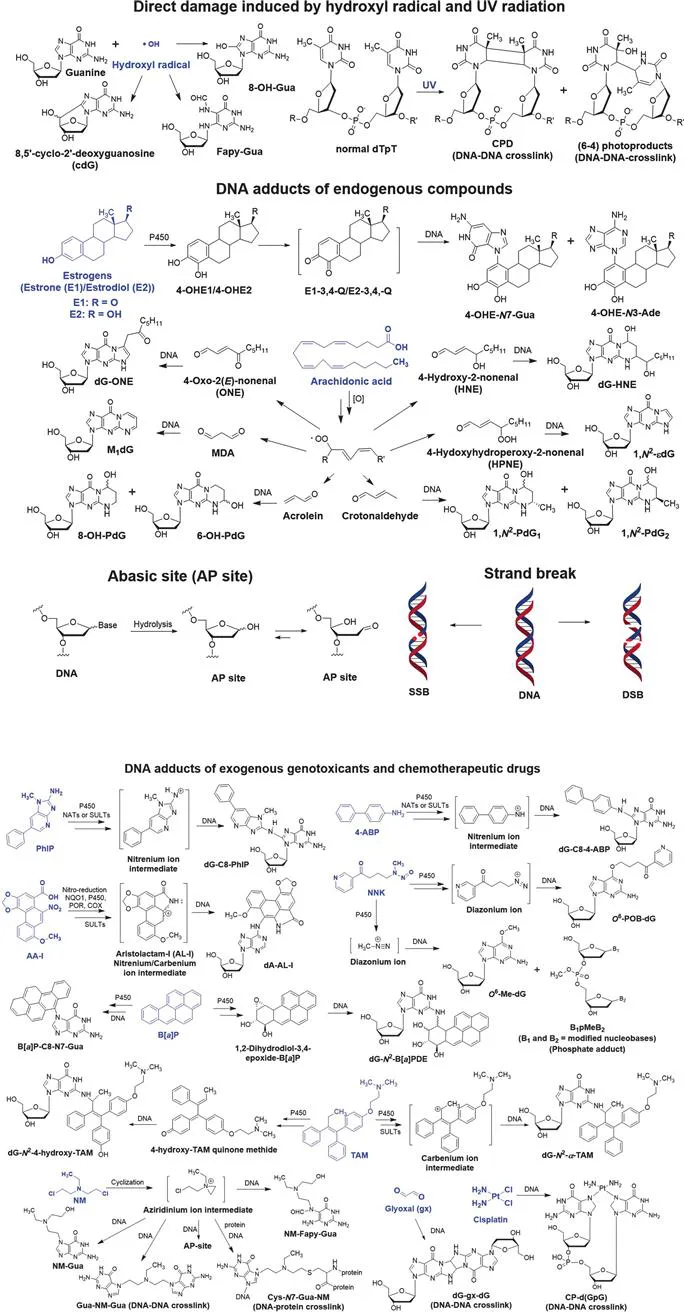
Humans are frequently exposed to low levels of hazardous chemicals in the environment, diet, and professional occupation, which damage the genome. DNA damage can also occur through biological processes, such as oxidative stress and inflammation, which produce reactive electrophiles. Thousands of lesions accumulate in the genome of the mammalian cell over 24 hours.1 Products of DNA damage include depurination or depyrimidination sites (abasic sites, or AP sites), covalent modifications (also known as adducts) of nucleobases and phosphate groups, intra- and interstrand cross-links, DNA–protein crosslinks, single-strand breaks (SSBs), and double-strand breaks (DSBs) (Figure 1.1).2 DNA repair enzymes efficiently remove many of these damages, however, some lesions may persist and induce mutations during cell division, leading to diseases including cancer. Furthermore, mutations in the genes encoding the proteins involved in DNA damage repair are linked to human diseases.3
Figure 1.1 Structures of endogenous chemicals, genotoxicants (highlighted in blue), their reactive intermediates, and DNA adduct products. The direct DNA damage arises from the hydroxyl radical (˙OH), an endogenous electrophile, and UV-radiation. Examples of endogenous and exogenous genotoxicants, and therapeutic drugs include the estrogen hormones; polyunsaturated fatty acids, such as arachidonic acid; PhIP, a heterocyclic aromatic amine and possible human prostate and colorectal carcinogen; B[a]P, a lung carcinogen; AA-I, a component in some herbal medicines and a kidney carcinogen; 4-ABP, a bladder carcinogen; tamoxifen, a chemotherapeutic drug used to treat breast cancer; cisplatin, a chemotherapeutic drug used to treat multiple cancers; NNK, a tobacco-specific nitrosamine and a lung carcinogen; bis(2-chloroethyl)-ethylamine, a chemotherapeutic nitrogen mustard.
Ionizing agents such as sunlight, hydroxyl radicals, and some genotoxicants directly damage DNA, but the vast majority of chemicals exert their genotoxicity through their metabolic reactive intermediates. Cytochrome P450s (P450s) are the most important enzymes involved in xenobiotic metabolism.4 P450s catalyze many reactions, including aliphatic and aromatic hydroxylation, N- or O-dealkylation, aliphatic desaturation, heteroatom oxidation, and epoxidation reactions. The resulting metabolites can contain functional groups such as –OH, –NH2, and –SH, some of which undergo conjugation reactions by UDP-glucuronosyltransferases (UGTs), sulfotransferases (SULTs), N-acetyltransferases (NATs), glutathione S-transferases (GSTs), and methyltransferases.5 Many xenobiotic metabolic reactions are considered as detoxification pathways since the formation of polar metabolites or conjugates facilitates the elimination of the chemicals through urine and feces. However, some metabolites or their conjugates are reactive intermediates. The N-oxidation of aromatic amines and heterocyclic aromatic amines (HAAs) leads to the formation of nitrenium ions,6 the oxidation of polycyclic aromatic hydrocarbons (PAHs) produces epoxides,7 the metabolism of cyclophosphamide and other prodrug nitrogen mustards (NM) lead to aziridinium ion intermediates,8 and the α-hydroxylation of N-nitroso compounds produces diazonium ions.9 These reactive intermediates covalently adduct to DNA and protein. Bis-electrophiles of some chemotherapeutic drugs and toxicants form protein–DNA and DNA–DNA cross-links that can block replication, transcription, and DNA repair processes.10
The identification of DNA adducts and their potential to induce mutations are among the first steps in the risk assessment of hazardous chemicals.11,12 DNA adducts are dosimeters for chemical exposure and a measure of the biologically effective dose. DNA adducts are critical biomarkers in risk assessment and aid in the formulation of public health policy, leading to the reduction of harmful chemical exposures. The totality of DNA damage of the genome is the DNA adductome, which is dynamic and the summation of recent and chronic exposures. The biomonitoring of DNA damage is most relevant when measured during the time that tumor formation commenced, rather than many years later when cancer is diagnosed. However, environmental pollution, tobacco smoking, and diet often represent long-term exposures, and current adduct levels of carcinogens from these exposures are likely to correlate with adduct levels that existed during the time of tumor initiation. Some DNA adducts may be linked to mutations in cancer-driver genes.13 DNA adducts also serve as biomarkers to assess the efficacy of anticancer drugs and may be used in precision medicine.14
Various biochemical and analytical techniques measure DNA damage. Some techniques analyze the modified DNA as an intact macromolecule. For example, gel electrophoresis is used to identify DNA cross-links, which migrate more slowly through the gel than non-modified DNA.15 The Comet assay detects the extent of DNA strand breaks based on the rapidly migrating DNA fragments or the length of the “comet-tail”.16 The aldehyde reactive probe and other alkoxyamines detect AP sites in the intact DNA.17 Immunodetection methods also screen macromolecular DNA for DNA damage.18,19 Other techniques hydrolyze DNA to the modified 2′-deoxynucleosides (dNs) or 2′-deoxynucleotides (nts). These DNA damage products are assayed by 32P-postlabeling, electrochemical detection (ECD), fluorescence, or mass spectrometry (MS). All methods that measure DNA damage require validation for accuracy, precision, linearity, reproducibility, and the mitigation of artifacts during the processing and analysis of DNA.