1.1 INTRODUCTION
There are 10 sources of energy and fuel on earth. These are coal, oil, gas, biomass, waste, nuclear energy, solar energy, wind energy, geothermal energy, and water. They can also be divided into three categories: fossil fuels (coal, oil, and natural gas), nuclear energy, and renewables (biomass, waste, solar energy, wind energy, geothermal energy, and water). Currently, about 85% of our energy needs are supplied by fossil fuels. Among fossil fuels, coal and oil have been the dominant sources for our fuel supply for power and transportation, respectively. In recent years, due to environmental concerns, while the use of coal for power production is declining, the use of gas for power production is on the rise. Use of natural gas in transportation industry is also slowly increasing.
Three fossil fuels, namely, coal, oil, and natural gas, were formed many hundreds of millions of years ago before the Age of Dinosaurs—hence the name “fossil fuels.” The age they were formed is called the Carboniferous (named after carbon) Period. It was part of the Paleozoic Era. The Carboniferous Period occurred from about 360 to 286 million years ago. At the time, the land was covered with swamps filled with huge trees, ferns, and other large leafy plants. The water and seas were filled with algae. Besides trees and vegetables, the remains of animals were also buried in the ground.
Natural gas is a fossil fuel formed when layers of buried plants, gases, and animals are exposed to intense heat and pressure over thousands of years. The energy that the plants originally obtained from the sun is stored in the form of chemical bonds in natural gas. Natural gas is a nonrenewable resource because it cannot be replenished on a human time frame. Natural gas is the gas component of coal, shale, oil, and water (in the form of clathrate hydrates under certain temperature and pressure conditions) formation. It is found with coal as coalbed methane (CBM), with oil as associated gas, with shale matrix as shale gas and as solid crystalline inclusion compounds, and with water as gas hydrates in arctic conditions and at the bottom of the sea. It can be used to generate heat, power, and liquid fuels and chemicals. It can be used for both static and mobile applications. Energy in 6000 cubic feet (ft3) of natural gas is equivalent to 1 barrel of oil.
While the high-velocity gas generates wind energy, this book is focused on the conversion of chemical energy from gas to heat, electricity, and liquid fuels. This chemical energy is generally obtained from natural gas recovered from underground or synthetic gas produced from a variety of nonrenewable and renewable sources.
The useful chemical constituents of natural and synthetic gas are largely methane, syngas (mixture of hydrogen and carbon monoxide), and hydrogen. Other lower hydrocarbons, ethane, and particularly propane and butane, are also used as fuels. Olefins such as ethylene and propylene are often used as raw materials to produce chemicals, polymers, etc. In this book we will mainly focus on various aspects of chemical constituents such as methane, syngas, hydrogen, propane (liquefied petroleum gas [LPG]), and butane and their roles in the energy and fuel industry. Collectively, these are called gaseous fuels. The scope of this book is to describe in detail the production, cleaning, upgrade, storage, and transport of gaseous fuels that occurred naturally and the ones that are man-made. The book also illustrates versatile applications of natural and synthetic gas for a variety of end products.
While both coal and oil contain a large number of aliphatic, olefin, and aromatic hydrocarbons, which can provide energy, the processing of coal and oil for heat and power productions also results in the production of harmful by-products and emissions. On the other hand, gas containing volatile hydrocarbons (mostly methane), syngas, and hydrogen provide fuel for energy in a cleaner form. Unlike coal and oil, natural or synthetic gas can be pretreated to produce high-quality pure methane, syngas, or hydrogen fuels, which can be used for numerous downstream applications. Both methane and hydrogen have high hydrogen to carbon ratios than coal or oil, making them cleaner and more efficient fuels. While the processing of coal and oil generates a significant amount of carbon dioxide, which is harmful to the environment, natural gas produces much lower carbon dioxide than coal during power production and much less harmful emissions than oil in heat and transportation applications. Harmful environmental effect by burning syngas is even lower and there is no carbon emission during the use of hydrogen.
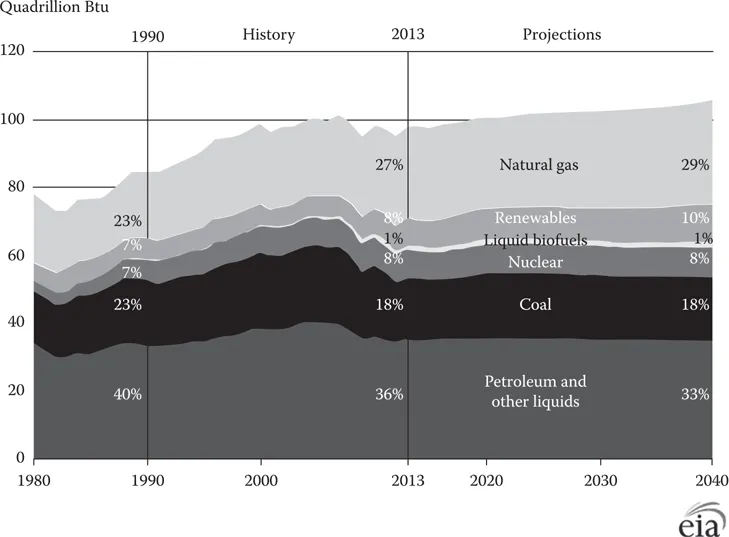
The average emissions rates in the United States from natural gas–fired power plants are 1135 lb/MWh of carbon dioxide, 0.1 lb/MWh of sulfur dioxide, and 1.7 lb/MWh of nitrogen oxides [1]. Thus, compared to the average air emissions from coal-fired generation, natural gas produces half as much carbon dioxide, less than a third as much nitrogen oxides, and 1% as much sulfur oxides at the power plant [1]. All new power plants in the United States are planning to use natural gas instead of coal. An Massachusetts Institute of Technology (MIT) study [2] indicates that the use of natural gas will be nearly doubled by 2040. Other projections shown in Figure 1.1 [3] indicate that natural gas will compete well with oil in the future.
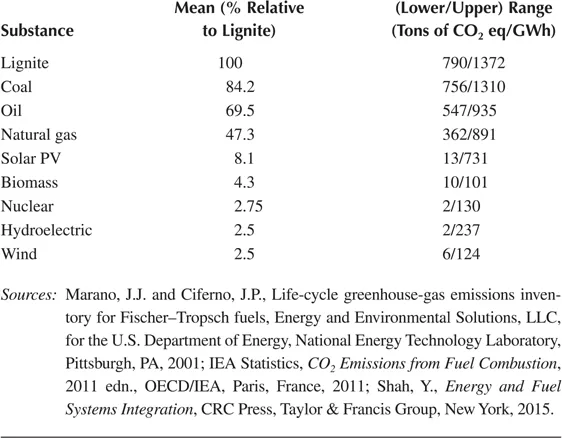
The increased share of natural gas in the global energy mix is not sufficient on its own to put the world on a carbon emission path consistent with an average global temperature rise of no more than 2°C. As shown in Table 1.1, natural gas is still not as good as renewable fuels and nuclear energy for its carbon emission. In the long term, more use of renewable energy and carbon-free nuclear energy is needed. The large-scale hydrogen production from natural or synthetic gas can, of course, make gas the ultimate source of fuel and energy along with renewable energy. At present time, nuclear power, renewable energy, and carbon capture and sequestration are relatively expensive next to gas. Until we make renewable energies and hydrogen commercially cheaper compared to fossil fuels, natural gas will remain the “transition fuel.”
TABLE 1.1
Relative Ranges of Greenhouse Gas Emissions from Different Electricity Generation Technologies
In general, natural gas is a mixture of several hydrocarbon gases, including methane (between 70% and 90%), ethane, propane, butane, and pentane, as well as carbon dioxide, nitrogen, and hydrogen sulfide. The composition of natural gas can vary widely, depending on the gas field. Natural gas is referred to as “wet” when hydrocarbons other than methane (particularly natural gas liquids [NGLs]) are present and “dry” when it is almost pure methane. Natural gas is also called “sour” when it contains significant amounts of hydrogen sulfide and “sweet” when it contains no sulfur.
Global gas demand was estimated at 3500 billion cubic meters (bcm) in 2013, which is up 1.2% from 2012 levels [4]. Gas demand has increased by around 800 bcm over the last decade, or 2.8% per year. Gas has a 21% share in the global primary energy mix, behind oil and coal. For comparison, 50 bcm of natural gas is roughly equivalent to 7% of U.S. consumption in 2012 or slightly more than Turkey’s entire annual consumption in 2012. The United States, Russia, China, and Iran are the world’s largest consumers of gas. The largest producers were Russia, the United States, Canada, Qatar, and Iran although this picture has changed in recent years due to the boom in shale gas production in the United States. It is important to note that Chinese gas consumption almost doubled over 2007–2012 and rose 9% in 2013 to reach nearly 120 bcm, while U.S. gas production increased by more than one-quarter over the same period to reach 688 bcm [4]. The United States consumed 19.7 million cubic feet (mcf) of natural gas in 1999, nearly all of which came from domestic production. Five states—Texas, Louisiana, Alaska, New Mexico, and Oklahoma—hold more than 85% of U.S. natural gas reserves. This picture is also significantly changed in recent years due to shale gas revolution.
In order to forecast the supply of natural gas, analysts mostly tend to refer to proven gas reserves, that is, volumes have been discovered and can be produced economically with existing technology at current gas prices. Worldwide proven conventional gas reserves are estimated at around 190 trillion cubic meters (tcm) or about 56 times the current annual global gas production. However, recoverable gas resources, that is, volumes that analysts are confident will be discovered or technology developed to produce them, are much larger, with recoverable conventional resources estimated at around 440 tcm [4, 5, 6 and 7]. Estimated recoverable unconventional resources (excluding methane hydrates) are around 240 tcm. Altogether, this would last around 220 years, based on the current rates of gas consumption [4, 5, 6 and 7].
Worldwide, many different units (for gas and associated energy supply and consumption) are used by different countries, which sometimes makes it difficult to reconcile data. At the Energy Information Administration (EIA) [8,9], natural gas statistics are given in bcm (for volume) and in terajoules (for energy). Worldwide, units such as kilowatt hours (kWh), kilocalories (kcal), million British thermal units (MBtu or MMbtu), therms (th), million tons of oil equivalent (mtoe), billion cubic feet (bcf), or billion cubic feet per day (bcf/d) are used. Data on liquefied natural gas (LNG) are given in tons or in bcm. It is worth noting that 1 m3 of LNG has much more energy than 1 m3 of gas at atmospheric pressure, due to their different physical states.
While the recent trend is to replace some of the fossil energy usage by renewable energy sources, within fossil energy the usage of coal and oil is also more substituted by gas both for heat and power productions. As mentioned earlier, this transformation is occurring due to more favorable impact by gas than coal or oil on environment. For example, the use of gas instead of coal can reduce CO2 emission by more than 50%–60% and in some cases as high as 80%. The use of LNG, LPG, and compressed natural gas (CNG) in heavy vehicle industry reduces CO2 emissions significantly compared to diesel fuel or gasoline. The automobile industry is making signif...