1
Endpoints for Cancer Clinical Trials
Stephen L. George
Xiaofei Wang
Herbert Pang
CONTENTS
1.1 Introduction
1.2 Overall Survival
1.3 Endpoints Based on Tumor Measurements
1.3.1 RECIST Criteria
1.3.2 Response Rate as Primary Endpoints
1.3.3 Tumor Response as Continuous Variable
1.4 Progression-free Survival and Other Composite Endpoints
1.5 Surrogate Endpoints
1.5.1 Definition
1.5.2 Surrogate Endpoint Validation
1.5.3 Remaining Issues
1.6 Patient-reported Outcomes
1.6.1 Patient-reported Outcomes
1.6.2 Types of PRO for Treatment Comparisons
1.6.3 Health Status, Functional and Symptoms Outcomes
1.6.4 General and Cancer-specific Quality of Life Outcomes
1.6.5 Criteria Used for PRO Instruments Selection
1.6.6 Reliability
1.6.7 Validity
1.6.8 Responsiveness of Instruments to Change
1.7 Promising New Approaches
1.7.1 Limitations of Traditional Endpoints
1.7.2 Pharmacokinetic and Pharmacodynamics Responses
1.7.3 Imaging Techniques
1.7.4 Immune Biomarkers-based Endpoints
1.7.5 Criteria for Evaluating Biomarker-based Endpoints
1.8 Summary
Acknowledgments
Bibliography
1.1 Introduction
The selection of appropriate endpoints for a clinical trial is an extremely important step in determining the appropriate design and analysis for the trial [106, 107]. An endpoint is ordinarily taken to mean an efficacy endpoint, a measure of the clinical efficacy of the treatments under consideration, although safety endpoints are also important. In this chapter we focus primarily on the key efficacy endpoints commonly used in cancer clinical trials, describing their strengths and weaknesses and, where appropriate, controversies surrounding their use.
In cancer trials, the most common practice is to specify a single primary endpoint and a primary trial objective based on this endpoint, used for setting the key design and analysis specifications. Other endpoints and objectives are usually relegated to secondary or exploratory roles. However, an increasingly common practice is to define co-primary endpoints and objectives, necessitating appropriate statistical adjustments for the resultant multiplicity [96].
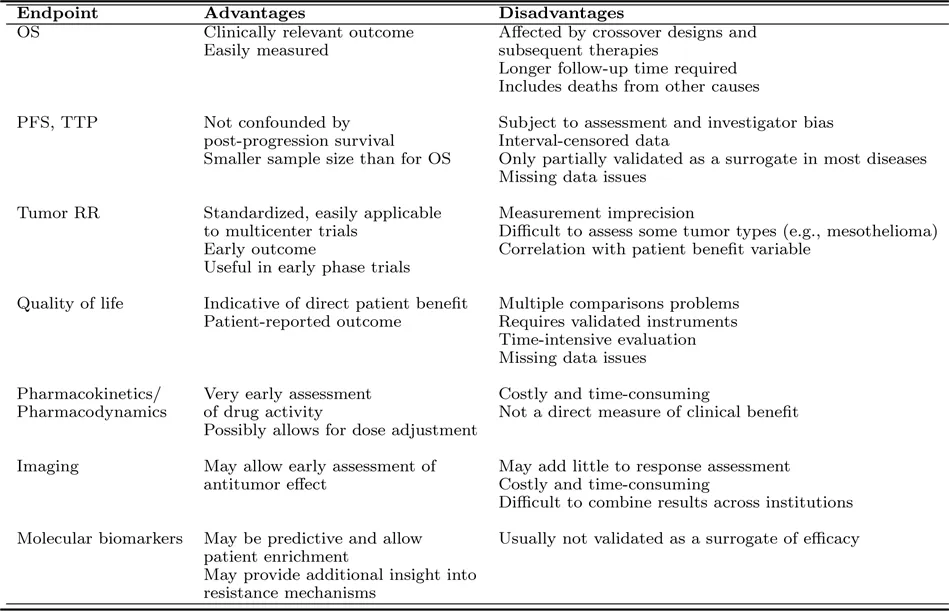
Examples of endpoints commonly used in cancer clinical trials include overall survival, generally agreed to be the gold standard efficacy endpoint; tumor response rate or other endpoints based on tumor measurements; composite endpoints such as progression-free survival and similar endpoints combining individual endpoints that may serve as surrogate endpoints for overall survival; patient-reported endpoints such as quality of life; and promising new approaches for defining endpoints including pharmacokinetic and pharmacodynamics responses, imaging techniques, and biomarker-based endpoints. These endpoints are summarized in Table 1.1 and are all discussed in more detail in the following sections of this chapter.
The particular endpoints chosen for a clinical trial will depend on many factors including the phase of the trial, the cost and feasibility of assessing the endpoint, the follow-up studies planned, and other factors. A simple statement of an endpoint to be used is not sufficient to define the specific aspect of the endpoint and the specific objectives that will be addressed in the trial. For example, comparing treatments via an endpoint such as overall survival or other time-to-event endpoints can be assessed in terms of the entire survival distribution, hazard ratios, median survival time differences, survival probabilities at some prespecified time point (e.g., 5 years), or other measures. The exact specification of the endpoint is an important detail and will determine key aspects of both design and analysis of the trial.
TABLE 1.1: Endpoints in Cancer Clinical Trials
There have been recent efforts by regulatory authorities and others to define the appropriate endpoints for trials of cancer treatment, both in general and for specific diseases. For example, the US Food and Drug Administration (FDA) has issued general guidelines for industry on endpoints appropriate for use when seeking regulatory approval for marketing of cancer drugs and biologics [98]. Specific FDA guidance documents for non-small cell lung cancer (NSCLC) and for imaging endpoints have also been published [100, 101]. Similarly, a European project entitled Definition for the Assessment of Time-to-event Endpoints in CANcer trials (DATECAN) aims to provide recommendations for time-to-event endpoints used in cancer clinical trials [10]. DATECAN guidelines for GIST tumors and pancreatic cancer have been recently published [9, 13]. Recommendations for hepatocellular carcinoma [63] and others have been published.
1.2 Overall Survival
Overall survival (OS), the time from randomization (for randomized trials) or from trial registration (for non-randomized trials) to death from any cause, is generally acknowledged as the gold standard endpoint for cancer clinical trials [76]. It reflects an obviously important clinical outcome, is easy to measure, and does not suffer from potential ascertainment or other biases existing for other endpoints. However, trials with objectives related to OS are generally quite large and may require a lengthy follow-up period. Surrogate endpoints for overall survival, discussed in detail below, are often used to reduce the size and duration of a planned trial.
In addition, there are other limitations and drawbacks in analysis and interpretation when using OS as a primary endpoint in cancer trials. For example, Phase I and II clinical trials are relatively small trials designed to determine an appropriate dose or schedule for an agent or to detect some minimal activity and, with rare exceptions, OS would in general not be an appropriate primary endpoint for such trials. Even for phase III cancer trials, in which OS is nearly always an important endpoint, there are difficulties. Most cancer therapies are given over a prolonged period of time, thereby increasing the probability of non-adherence to the assigned treatment. Even with excellent adherence to the originally assigned treatment regimen, cancer patients typically move through various disease states prior to death (e.g., patients may experience a disease...