
- 274 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
About this book
The second edition of Metal Ions in Biochemistry deals with the multidisciplinary subject of bio-inorganic chemistry, encompassing the disciplines of inorganic chemistry, biochemistry and medicine.
The book deals with the role of metal ions in biochemistry, emphasising that biochemistry is mainly the chemistry of metal-biochemical complexes. Hence, the book starts with the structures of biochemicals and the identification of their metal binding sites. Thermodynamic and kinetic properties of the complexes are explained from the point of view of the nature of metal-ligand bonds. Various catalytic and structural roles of metal ions in biochemicals are discussed in detail.
Features
-
- The role of Na+ and K+ in brain chemistry.
-
- The role of zinc insulin in glucose metabolism and its enhancement by vanadium and chromium compounds.
-
- Discussion of the role of zinc signals, zinc fingers and cascade effect in biochemistry.
-
- Haemoglobin synthesis and the role of vitamin B12 in it.
-
- The role of lanthanides in biochemical systems.
-
- A detailed discussion of the role of non-metals in biochemistry, a topic missing in most of the books on bio-inorganic chemistry.
The study of bio-inorganic chemistry makes biochemists rethink the mechanistic pathways of biochemical reactions mediated by metal ions. There is a realisation of the role of metal complexes and inorganic ions as therapeutics such as iron in leukaemia, thalassemia and sickle cell anaemia, iodine in hypothyroidism and zinc, vanadium and chromium in glucose metabolism. The most recent realisation is of the use of zinc in the prevention and treatment of COVID-19.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Structure of Cells and Introduction to Bioinorganic Chemistry
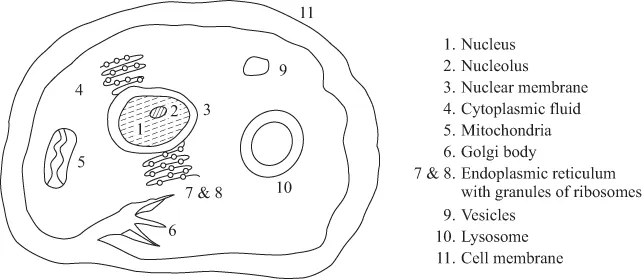
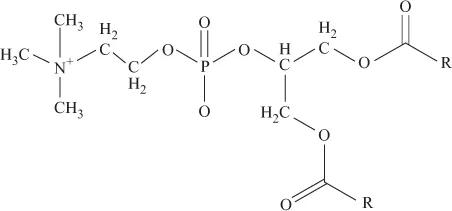
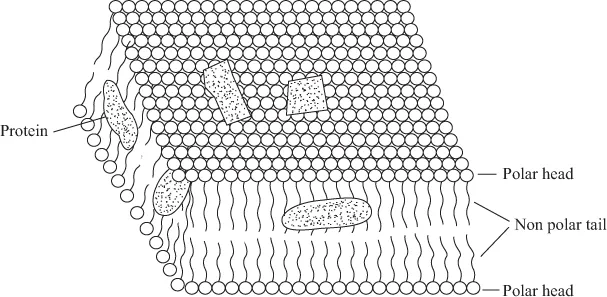
Biomolecules and Their Metal Coordination Behaviour
- Sugars: They serve as source of energy in biochemical systems. Oxidation of glucose in the respiration liberates energy and sustains life. Hexose sugar (C6H12O6) occurs in the form of aldohexoses (glucose, galactose, mannose being more important, having six-membered ring structure) and ketohexose (fructose, having five-membered ring structure) (Figure 1.4).
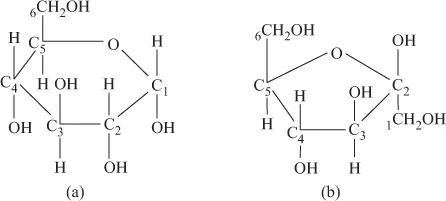 FIGURE 1.4 (a) Aldohexose. (b) Ketohexose.Various hexoses condense, forming glycosidic linkages, and result in polysaccharides (C6H10O5)n,e.g. starch and cellulose.Pentose sugar ribose (C5H10O5) and deoxyribose (C5H10O4) are the constituents of RNA and DNA, respectively (Figure 1.5).
FIGURE 1.4 (a) Aldohexose. (b) Ketohexose.Various hexoses condense, forming glycosidic linkages, and result in polysaccharides (C6H10O5)n,e.g. starch and cellulose.Pentose sugar ribose (C5H10O5) and deoxyribose (C5H10O4) are the constituents of RNA and DNA, respectively (Figure 1.5).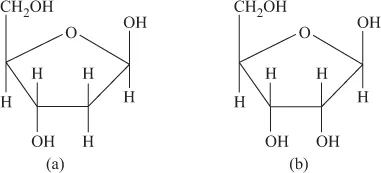 FIGURE 1.5 (a) Deoxyribose. (b) Ribose.Sugars have weak binding tendency with metal ions. Weak complexes are formed by the coordination of sugars from –OH sites. Mainly, sugar complexes of hard acid metal ions, like Ca2+, are known. The structure of Ca(II) complex of inositol is shown in Figure 1.6.
FIGURE 1.5 (a) Deoxyribose. (b) Ribose.Sugars have weak binding tendency with metal ions. Weak complexes are formed by the coordination of sugars from –OH sites. Mainly, sugar complexes of hard acid metal ions, like Ca2+, are known. The structure of Ca(II) complex of inositol is shown in Figure 1.6.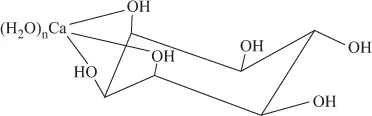 FIGURE 1.6 Calcium complex of inositol.Allose, lactose and ribose form relatively more stable complexes, but are not abundant in nature. The gel formation of the polysaccharide alginic acid, in the presence of Ca, is attributed to the binding of the Ca(II) ions to the –OH and –O groups of the two constituent molecules, β-d-mannuronic acid and α-l-gluconic acid, of the polysaccharide.
FIGURE 1.6 Calcium complex of inositol.Allose, lactose and ribose form relatively more stable complexes, but are not abundant in nature. The gel formation of the polysaccharide alginic acid, in the presence of Ca, is attributed to the binding of the Ca(II) ions to the –OH and –O groups of the two constituent molecules, β-d-mannuronic acid and α-l-gluconic acid, of the polysaccharide. - Amino acids: Amino acids are the basic constituents of the polypeptides and proteins. Essentially, they have an acidic –COOH group and a basic –NH2 group and hence in neutral solution exist as zwitterion (Figure 1.7a).
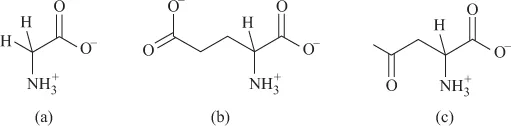 FIGURE 1.7 (a) Glycine. (b) Glutamic acid. (c) Aspartic acid.In the case of polycarboxylic amino acids (glutamic acid, aspartic acid), there are additional acidic sites (Figure 1.7a, b and c), whereas the amino acids histidine and arginine have additional basic imidazole or secondary amine sites, respectively (Figure 1.8a and b).
FIGURE 1.7 (a) Glycine. (b) Glutamic acid. (c) Aspartic acid.In the case of polycarboxylic amino acids (glutamic acid, aspartic acid), there are additional acidic sites (Figure 1.7a, b and c), whereas the amino acids histidine and arginine have additional basic imidazole or secondary amine sites, respectively (Figure 1.8a and b).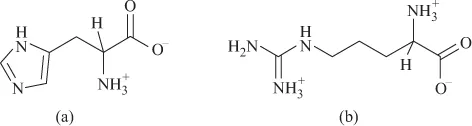 FIGURE 1.8 (a) Histidine. (b) Arginine.The ionic properties of amino acids are responsible for their buffering activity in biological systems.Amino acids are strongly coordinating molecules, binding to the metal ion from NH2 and COO− sites, resulting in the formation of chelate rings.The formation constants of the metal complexes should increase with the basicity of the amino acid, that is proton binding capacity of NH2 site.If the amino acid has additional coordinating sites, it can form more stable complex. For example, aspartic acid has an additional acidic carboxylic group. It forms more stable complex, as there is coordination from two carboxylates and NH2 site, resulting in greater stability (Figure 1.9a).
FIGURE 1.8 (a) Histidine. (b) Arginine.The ionic properties of amino acids are responsible for their buffering activity in biological systems.Amino acids are strongly coordinating molecules, binding to the metal ion from NH2 and COO− sites, resulting in the formation of chelate rings.The formation constants of the metal complexes should increase with the basicity of the amino acid, that is proton binding capacity of NH2 site.If the amino acid has additional coordinating sites, it can form more stable complex. For example, aspartic acid has an additional acidic carboxylic group. It forms more stable complex, as there is coordination from two carboxylates and NH2 site, resulting in greater stability (Figure 1.9a).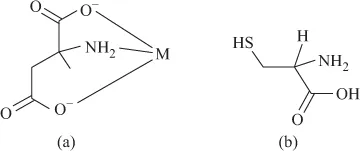 FIGURE 1.9 (a) Aspartate. (b) Cysteine.Cysteine, with an additional SH coordinating site, is ambidentate in character (Figure 1.9b). It can coordinate from NH2 and SH sites or from NH2 and COO− sites. It has, however, been confirmed that the coordination is of the former type, as the formation constant of Zn(II) cysteine complex is comparable with that of ...
FIGURE 1.9 (a) Aspartate. (b) Cysteine.Cysteine, with an additional SH coordinating site, is ambidentate in character (Figure 1.9b). It can coordinate from NH2 and SH sites or from NH2 and COO− sites. It has, however, been confirmed that the coordination is of the former type, as the formation constant of Zn(II) cysteine complex is comparable with that of ...
Table of contents
- Cover
- Half Title
- Title Page
- Copyright Page
- Dedication
- Table of Contents
- Preface to First Edition
- Preface to Second Edition
- Acknowledgements
- Authors
- 1. Structure of Cells and Introduction to Bioinorganic Chemistry
- 2. Thermodynamic and Kinetic Properties of Metal Complexes
- 3. Alkali and Alkaline Earth Metal Ions in Biochemical Systems
- 4. Zinc in Biochemical Systems
- 5. Iron in Biochemical Systems
- 6. Copper in Biochemical Systems
- 7. Cobalt in Vitamin B12 in Biochemical System
- 8. Molybdenum in Nitrogen Fixation in Plants
- 9. Magnesium and Manganese in Photosynthesis in Plants
- 10. Less Common Trace Metal Ions in Biochemical Systems
- 11. Metal Ion Toxicity in Biochemical Systems
- 12. Metal Complexes in Therapeutics
- 13. Role of Trace Nonmetals in Biochemical Systems
- Answers
- Suggested Reading
- Index