
Real-World Evidence in Drug Development and Evaluation
- 208 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Real-World Evidence in Drug Development and Evaluation
About this book
Real-world evidence (RWE) has been at the forefront of pharmaceutical innovations. It plays an important role in transforming drug development from a process aimed at meeting regulatory expectations to an operating model that leverages data from disparate sources to aid business, regulatory, and healthcare decision making. Despite its many benefits, there is no single book systematically covering the latest development in the field.
Written specifically for pharmaceutical practitioners, Real-World Evidence in Drug Development and Evaluation, presents a wide range of RWE applications throughout the lifecycle of drug product development. With contributions from experienced researchers in the pharmaceutical industry, the book discusses at length RWE opportunities, challenges, and solutions.
Features
- Provides the first book and a single source of information on RWE in drug development
- Covers a broad array of topics on outcomes- and value-based RWE assessments
- Demonstrates proper Bayesian application and causal inference for real-world data (RWD)
- Presents real-world use cases to illustrate the use of advanced analytics and statistical methods to generate insights
- Offers a balanced discussion of practical RWE issues at hand and technical solutions suitable for practitioners with limited data science expertise
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1
Using Real-World Evidence to Transform Drug Development: Opportunities and Challenges
1.1 Introduction
1.2 Traditional Drug Development Paradigm
1.2.1 Drug Development Progress
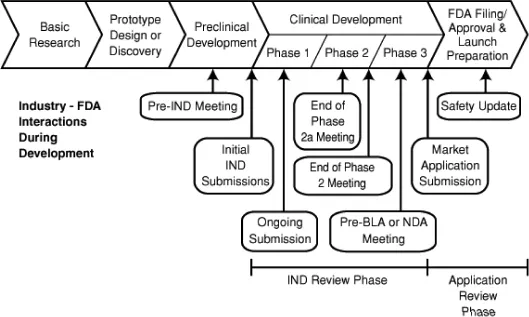
Drug development process. Adopted from FDA website.
1.2.2 Limitations of Traditional Randomized Controlled Trials
1.3 Real-World Data and Real-World Evidence
1.3.1 Real-World Data
Table of contents
- Cover
- Half Title
- Series Page
- Title Page
- Copyright Page
- Contents
- Preface
- 1. Using Real-World Evidence to Transform Drug Development: Opportunities and Challenges
- 2. Evidence Derived from Real-World Data: Utility, Constraints, and Cautions
- 3. Real-World Evidence from Population-Based Cancer Registry Data
- 4. External Control Using RWE and Historical Data in Clinical Development
- 5. Bayesian Methods for Evaluating Drug Safety with Real-World Evidence
- 6. Real-World Evidence for Coverage and Payment Decisions
- 7. Causal Inference for Observational Studies/Real-World Data
- 8. Introduction to Artificial Intelligence and Deep Learning with a Case Study in Analyzing Electronic Health Records for Drug Development
- Index