The Atmosphere
Key Terms
absolute humidity
atmospheric pressure
barometer
density altitude
dew point
exosphere
humidity
International Standard Atmosphere
ionosphere
lapse rate
mercury
ozone
pressure altitude
relative humidity
standard datum plane
stratosphere
tropopause
troposphere
U.S. Standard Atmosphere
vapor pressure
water vapor
Symbols & Abbreviations
| h | altitude (feet) |
| Hd | density altitude |
| P | pressure (lb/ft2) |
| T | temperature |
| ρ | air density (slugs per cu. ft) |
Introduction
To begin understanding how an airplane flies, a pilot should be familiar with the basic principles of the atmosphere. This chapter explains the composition of the atmosphere, the basics of atmospheric pressure, and water content. These basic principles and physical laws provide the foundation a pilot needs in order to have an understanding of aerodynamics for airplanes.
The atmosphere is a gaseous envelope covering the earth. If the earth were the size of a basketball, the thickness of the atmosphere would be about as thick as a pillowcase wrapped tightly around the ball. Gravity holds the atmosphere to the earth’s surface as it rotates. The atmosphere also has motions called circulations that occur relative to the earth’s surface. Circulations are caused primarily by the temperature differential that exists between the tropic and the polar regions, and by uneven heating of land and water areas by the sun.
Heavier-than-air flight is dependent upon the aerodynamic force originating from a category of fluids known as gases. The earth’s atmosphere is a mixture of gases with traces of water vapor and various other components such as argon and carbon dioxide. The magnitudes of the aerodynamic forces acting on an aircraft are dependent on the shear stress and pressures exerted by the gases that surround it.
Characteristics of the atmosphere such as pressure, temperature, and velocity are used by a pilot to determine whether a flight will achieve adequate performance, or even become airborne. The characteristics of the atmosphere vary with altitude, and each characteristic has a unique effect on weather and many other detailed data points that are also considered in the preparation of flight plans.
Blaise Pascal (1623–1662) and Evangelista Torricelli (1608–1647) have been credited with developing the barometer, an instrument for measuring atmospheric pressure. The results of their experiments are still used today with very little improvement in design or knowledge. They determined that air has weight which changes as altitude is changed with respect to sea level. Today scientists are also interested in how the atmosphere affects the performance of an aircraft and its equipment.
Composition of the Atmosphere
The atmosphere is an envelope of air that surrounds the earth and rests upon its surface. It is as much a part of the earth as the sea or land, but air differs from land and water in that it is a mixture of gases. The atmosphere has mass, weight, and an indefinite shape.
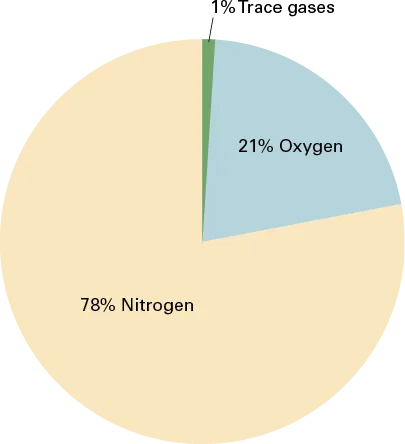
All matter is constructed of atoms, with the configuration of the atom determining the kind of matter present (e.g., oxygen, neon, silver). Individual atoms can combine with other atoms to form molecules. Under normal conditions, matter on Earth exists as a solid, a liquid, or a gas. The atmosphere consists of a mixture of various gases. Dry air is composed of approximately 78 percent nitrogen, 21 percent oxygen, and a 1 percent mixture of other gases, mostly argon (Figure 1-1). Some of these elements are heavier than others. Heavier elements, such as oxygen, settle to the surface of the earth, while lighter elements rise up to the higher-altitude regions. Most of the atmosphere’s oxygen is contained below 35,000 feet. Air, like a fluid, is able to flow and change shape when subjected to even minute changes in pressure because it lacks strong molecular cohesion. For example, gas will completely fill any container into which it is placed, expanding or contracting to adjust its shape to the limits of the container.
One of the important components of the atmosphere is water vapor, which varies in amounts from 0 to 5 percent by volume. It is present in three physical states: as a gas, liquid, and solid. The maximum amount of gaseous water vapor the air can hold is temperature-dependent; the higher the temperature, the more water vapor the air can hold. Water vapor remains invisibly suspended in the atmosphere until, through condensation, it grows to sufficient droplet or ice crystal size to form clouds or precipitation.
In addition to a number of gases, the atmosphere also contains variable quantities of foreign matter and impurities such as pollen, dust, bacteria, soot, volcanic ash, spores, salt particles, and dust from outer space—even when the air is apparently clear.
Figure 1-1. Composition of the atmosphere.
The atmosphere is a complex and ever-changing mixture. Its ingredients vary from place to place and from day to day. The composition of the air remains almost constant from sea level up to its highest level, but its density diminishes rapidly with altitude. Six miles up, for example, the air is too thin to support respiration, and 12 miles up, not enough oxygen is present to support combustion, except in some specially designed turbine engine-powered airplanes.
At a point several hundred miles above the earth, some gas particles spray out into space, some of which are dragged by gravity and fall back into the air below, while others never return. Physicists disagree as to the boundaries of the outer fringes of the atmosphere. Some think it begins 240 miles above the earth and extends to 400 miles; others place its lower edge at 600 miles and its upper boundary at 6,000 miles. Certain nonconformities exist at various levels. Between 12 and 30 miles above the earth, high solar ultraviolet radiation reacts with oxygen molecules to produce a thin curtain of ozone (O3), which is a very poisonous gas—but one without which life on earth could not exist. The ozone layer filters out a portion of the sun’s lethal ultraviolet rays, allowing only enough through to give us sunburn, kill bacteria, and prevent rickets.
At 50 to 65 miles up, most of the oxygen molecules begin to break down under solar radiation into free atoms, and form hydroxyl ions (OH) from water vapor. In this region, all the atoms also become ionized.
Studies of the atmosphere have revealed that the temperature does not decrease uniformly with increasing altitude. Instead, it gets steadily colder up to a height of about 7 miles, where the rate of temperature change slows down abruptly and remains almost constant at −55 degrees Centigrade (218 Kelvin) up to about 20 miles. Then the temperature begins to rise to a peak value of 77°C (350 Kelvin) at the 55-mile level. Thereafter, it climbs steadily until reaching 2,270°C (2,543 K) at a height of 250 to 400 miles. From the 50-mile level upward, a human or any other living creature, without the protective cover of the atmosphere, would be broiled on the side facing the sun and frozen on the other.
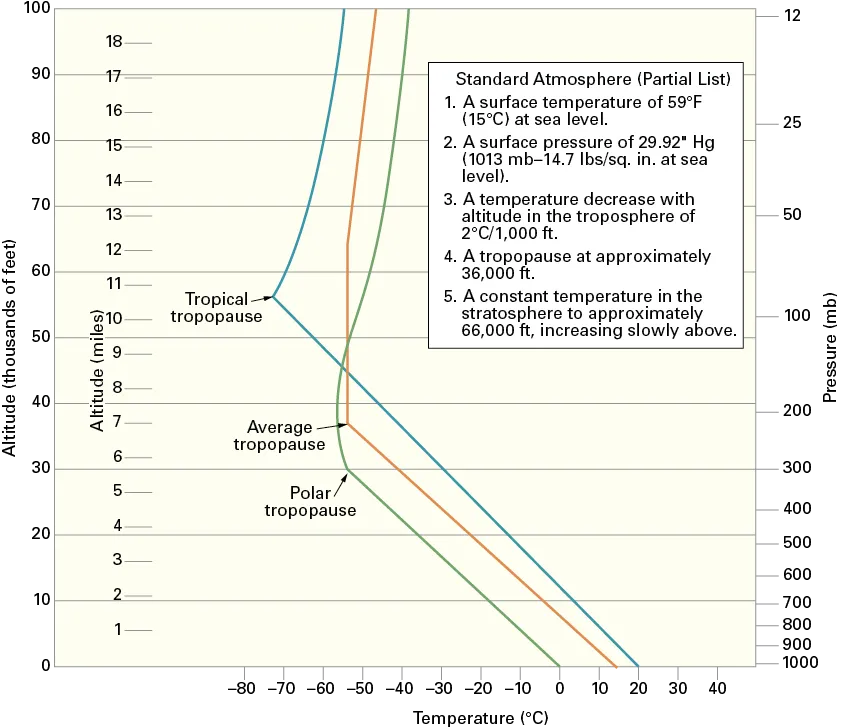
The atmosphere is divided into four concentric layers or levels: the troposphere, stratosphere, ionosphere, and exosphere. Transition through these layers is gradual and without sharply defined boundaries. However, one boundary, called the tropopause, exists between the first and second layer.
Figure 1-2. The height of the tropopause varies with latitude. (U.S. Airforce)
The troposphere extends from the earth’s surface to about 35,000 feet at middle latitudes, but varies from 28,000 feet at the poles to about 54,000 feet at the equator (Figure 1-2). The troposphere is characterized by large changes in temperature and humidity and by generally turbulent conditions. Nearly all cloud formations are within the troposphere, and approximately three-fourths of the total weight of the atmosphere is within the troposphere. The troposphere is defined by a decrease in temperature with an increase in altitude. As a general rule of thumb, localized inversions can cause the temperature to increase with altitude.
The troposphere and stratosphere are separated by the tropopause. The tropopause is defined as the point in the atmosphere at which the decrease in temperature (with increasing altitude) abruptly ceases.
The stratosphere extends from the upper limits of the troposphere (and the tropopause) to an average altitude of 60 miles. The upper portion of the stratosphere is often called the chemosphere or ozonosphere. The stratosphere is characterized by level temperature and a very stable atmosphere. Because the temperature in the tropopause and lower stratosphere remains constant (or slightly decreases) with increasing altitude, very little convective turbulence occurs at these altitudes. Though most turbulence at this altitude is caused by variations in the jet stream and other local wind shears, areas of significant convective activity (thunderstorms) occur below the stratosphere, in the troposphere.
The ionosphere ranges from the 50-mile level to a level ...