
Industrial Green Chemistry
- 297 pages
- English
- ePUB (mobile friendly)
- Available on iOS & Android
Industrial Green Chemistry
About this book
The editors and authors, with backgrounds in academia and industry, tie together recent and established technologies for the upcoming change to sustainable industrial chemistry. The extensive worldwide activities towards that goal are exemplified with a series of green processes. Some of these processes are already commercially applied (squalene to squalane, hydraulic fluids from vegetable oils, biosourced polycarbonates), others are ready for a large scale implementation (glycerol to acrylic acid, biosourced acrylonitrile and levulinic acid, polyamides from fatty nitriles-esters hydrogenation, butadiene from bioethanol) or are being developed (cyclic carbonates from epoxides, selective pyrolysis of biomass). This book is an indispensable source for the researchers and professionals who work for a greener chemical industry. The chapters have been arranged to guide students through the design of new processes for more sustainable chemistry, using case studies as examples.
Frequently asked questions
- Essential is ideal for learners and professionals who enjoy exploring a wide range of subjects. Access the Essential Library with 800,000+ trusted titles and best-sellers across business, personal growth, and the humanities. Includes unlimited reading time and Standard Read Aloud voice.
- Complete: Perfect for advanced learners and researchers needing full, unrestricted access. Unlock 1.4M+ books across hundreds of subjects, including academic and specialized titles. The Complete Plan also includes advanced features like Premium Read Aloud and Research Assistant.
Please note we cannot support devices running on iOS 13 and Android 7 or earlier. Learn more about using the app.
Information
1 Conversion of glycerol to acrylic acid
Abstract
1.1 Introduction
- low cost
- commercial capacity (due to increased biodiesel production) and
- worldwide availability
1.2 Glycerol and its applications
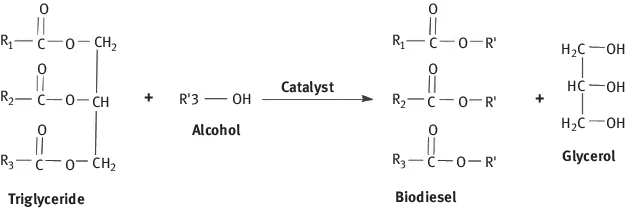
Table of contents
- Title Page
- Copyright
- Contents
- 1 Conversion of glycerol to acrylic acid
- 2 Alternative routes to more sustainable acrylonitrile: biosourced acrylonitrile
- 3 Biobased levulinic acid production
- 4 Fatty nitrile esters hydrogenation for biosourced polyamide polymers
- 5 Ni-free hydrogenation of natural products for the personal care industry: case study, squalene hydrogenation
- 6 High-performance hydraulic fluids from vegetable oils
- 7 Biomass valorization: bioethanol upgrading to butadiene
- 8 Biosourced polycarbonates
- 9 Organic cyclic carbonates synthesis under mild conditions
- 10 Biomass selective pyrolysis, bio-oil separation and products development: challenges and opportunities for green chemistry
- Index